This is a revised version of a previous analogy. The original version is here.
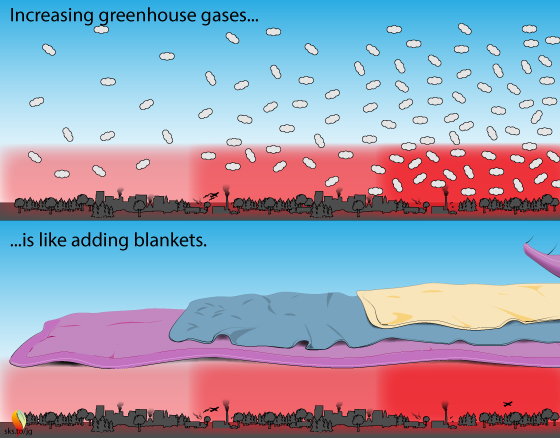
The greenhouse effect is like a stack of blankets on a winter night.
More blankets = more warmth: The greenhouse effect is like blankets warming the Earth. If it is 10°C (50°F) in your bedroom, you need a few blankets to keep yourself warm. More blankets = more warming. Too many blankets and you sweat. The greenhouse effect is a good thing, up to a point.

For the atmospheric temperature to be stable (i.e., no global warming nor cooling), the Earth must lose just as much energy to space as it gains from the sun. How does this work? If global warming is happening, what will ever allow it to stop and for atmospheric temperatures to stop rising?
The sun sends us more energy that we can use, in the form of a broad spectrum of light. Earth absorbs about 70% of the incident light, and reflects the rest out to space. The 70% of the suns energy that is absorbed heats the Earth. Although the energy that comes from the sun is in the form of a broad spectrum of wavelengths, things on Earth predominantly re-emit infrared radiation. Infrared radiation is emitted in all directions, including skyward. When you are sitting next to a fire, you feel the infrared heat from the fire, and some of that infrared heat moves upward, towards space. Does it make it to space?
There are many things that may prevent infrared radiation from making it to space: a passing airplane may absorb some, clouds, and some gases in the atmosphere are particularly effective at capturing infrared radiation: the so-called greenhouse gases (GHG's). The more GHG's we pump into the atmosphere, the more infrared radiation it traps, and the more the atmosphere heats up. This is global warming. But if the atmosphere warms up when we add more GHG's, what limits the amount of warming?
When things heat up they emit more infrared radiation. Compare the heat emitted from a smoldering fire to that from a roaring fire. As the atmosphere warms up, it emits more infrared radiation, some of it skyward. The warmer the atmosphere, the more infrared radiation is emitted. Although GHG's inhibit the motion of infrared radiation through the atmosphere, a warmer atmosphere forces more infrared radiation up and out into space. Eventually a balance is reached where a warmer atmosphere manages to force enough infrared radiation up and out into space to reject to space the same amount of solar energy absorbed by the Earth.
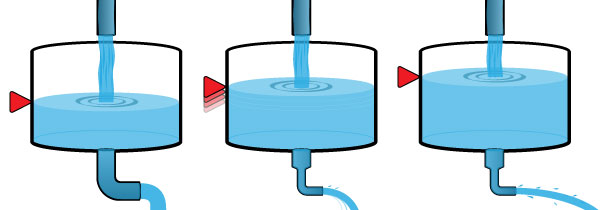
To visualize how this works, imagine a tank, with water flowing in and out. The water level is balanced when the flow rate into the tank equals the flow rate out of the tank. What happens if we restrict the outflow (middle picture above)? This is comparable to how GHG's restrict the flow of infrared radiation through the atmosphere. Initially the outflow rate decreases, causing less water to flow out than into the tank. The water level rises in response. This is comparable to atmospheric temperature increasing. But a higher water level applies more pressure to the outlet, increasing the outflow rate. As the level of water rises in the tank, the outlet flow rate increases, until a new balance is reached between inflow and outflow. The result of the restriction on the outlet is a higher water level in the tank, but otherwise the inflow and outflow rates are the same as at the beginning.
Although some of the details here might seem complicated and cause head scratching, the basic concept is simple: adding GHG's to the atmosphere is like adding more insulating blankets on top of you: you get warmer.
Replete with fancy graphics, high-tech videos, and communicated across social-media channels, it often feels as though climate science is a product of our modern age: the thing driving GHG emissions. But crack open a dusty journal from days gone by, and we soon realize that our knowledge about the "warming blankets" over Earth dates back to the dawn of the Industrial Revolution.
In 1858 a female American researcher, Eunice Foote, published the following in The American Journal of Science and Arts (Vol XXII, Nov. 1856)
“The highest effect of the sun’s rays I have found to be in carbonic acid gas [CO2]. ... An atmosphere of that gas would give to our earth a high temperature; and if as some suppose, at one period of its history, the air had mixed with it a larger proportion than at present, an increased temperature from its own action, as well as from increased weight, must have necessarily resulted.”
Five years later John Tyndall, the person generally recognized as discovering the heat-trapping characteristics of CO2 and other GHG's, wrote the following in the Philosophical Transactions of the Royal Society of London (Vol. 151 (1861), pp. 1-36)
“Now if, as the above experiments indicate, the chief influence be exercised by the aqueous vapour, every variation of this constituent must produce a change of climate. Similar remarks would apply to the carbonic acid [CO2] diffused through the air; while an almost inappreciable admixture of any of the hydrocarbon vapours would produce great effects on the terrestrial rays and produce corresponding changes of climate.”
We've known for a very long time about the heat-trapping effect of GHG's and that they act like a pile of blankets over the Earth.
Posted by Evan on Wednesday, 9 November, 2022
 |
The Skeptical Science website by Skeptical Science is licensed under a Creative Commons Attribution 3.0 Unported License. |