On February 14, 2023 we announced our Rebuttal Update Project. This included an ask for feedback about the added "At a glance" section in the updated basic rebuttal versions. This weekly blog post series highlights this new section of one of the updated basic rebuttal versions and serves as a "bump" for our ask. This week features "What is methane's contribution to global warming?". More will follow in the upcoming weeks. Please follow the Further Reading link at the bottom to read the full rebuttal and to join the discussion in the comment thread there.
Just like CO2, methane is a colourless, odourless gas. But the similarity ends there. Methane is highly reactive, to the extent that it can form a highly explosive mixture with oxygen. Methane explosions are a leading cause of mining disasters. For domestic use, the gas has an odour-producer added to it, so you can 'smell gas', in the event of a leak.
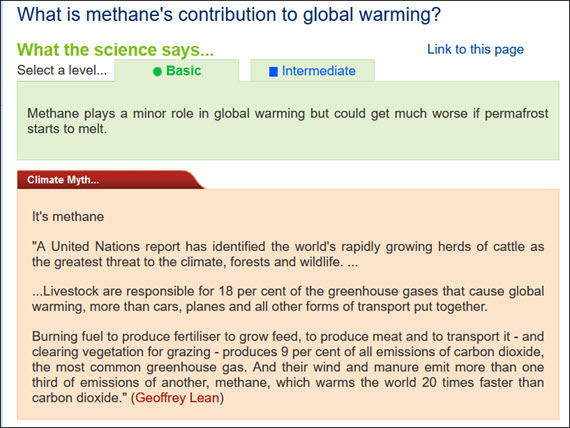
That reactivity is a good thing, since methane is a potent greenhouse gas, many times more effective at trapping heat in the atmosphere than CO2. It has caused almost a third of recent global warming. But methane oxidises quickly. The atmospheric lifetime of a molecule of methane is typically no more than 12 years. This is much shorter than the long atmospheric lifetime of CO2.
Due to that reactivity, the concentration of methane in the atmosphere is much smaller than that of CO2. For that reason, it is expressed in parts per billion (ppb). A thousand parts per billion is one part per million (ppm). Currently, the average methane concentration is 1894 ppb or 1.894 ppm. That is about 2.5 times pre-industrial levels.
Current sources of methane are a mixture of natural and manmade processes, according to the International Energy Agency. Man-made sources make up more than two thirds of the total. The key natural source is wetlands. However, there is also the poorly-understood potential for releases from methane hydrate deposits.
Methane hydrate, or methane clathrate as it's sometimes called, is a white, snow-like solid. Although it looks like snow, the resemblance ends there, because you can set fire to it. Methane hydrate occurs in marine sediments, where it forms during the bacterial decomposition of organic matter. Vast stores of the substance can build up in the sediment, over time.
Importantly though, methane hydrate is only stable at very high pressures and low temperatures. Such environments are typically found within the slopes that lead down from the continental shelves into the oceanic depths. Here, the water is deep and cold enough and there is still plenty of organic matter too. For methane hydrate, the conditions are perfect. Destabilisation of this buried 'flammable snow' could lead to methane release on a substantial scale. But it's important to bear in mind that this remains an incompletely-understood area - despite occasional scary headlines in the media. Vast-scale methane-release is regarded as very unlikely to occur under any plausible near-term emissions pathway.
Methane outgassing from melted permafrost is much better understood. You may have seen videos of methane ignition at lakes in permafrost-country. But currently, compared to man-made sources, this is still insignificant in the great scheme of things.
The leading source of man-made methane emissions is agriculture but the energy sector comes a close second. Waste treatment, in particular landfill, is also significant. Improvements are possible in all such sectors and are in some cases being implemented. Meanwhile, emitting CO2 at the rate of over 40 billion tons per annum, as we are now doing, still remains a seriously bad Idea. Methane should not distract us from that.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
In case you'd like to explore more of our recently updated rebuttals, here are the links to all of them:
If you think that projects like these rebuttal updates are a good idea, please visit our support page to contribute!
Posted by John Mason on Tuesday, 26 December, 2023
 |
The Skeptical Science website by Skeptical Science is licensed under a Creative Commons Attribution 3.0 Unported License. |