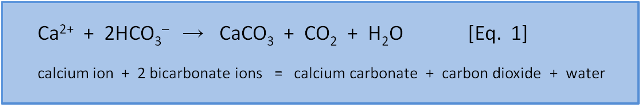
This is the 3rd post in our Ocean Acidification is not OK series. Other posts: Introduction , 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.
In the last post we introduced thermodynamics and explained how it could predict a reaction direction. In this post we will describe why making calcium carbonate shells via Equation 1 is so easy.
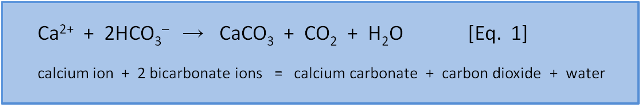
Calcium carbonate, CaCO3, is a salt. A salt is a substance containing an ion with a positive electric charge, (cation - like calcium Ca2+ or sodium Na+) and an ion with a negative electric charge (anion - like carbonate CO3– or chloride Cl–). Thus, sodium chloride or table salt, NaCl, contains Na+ and Cl–. While Equation 1 reminds us that while CaCO3 contains the ions calcium Ca2+ and carbonate CO32–, the actual reaction to make it is more complicated than calcium + carbonate = calcium carbonate.
In chemistry precipitation is the process when a solid (often a salt) forms from a solution. One familiar example is the way crystals of sodium chloride, NaCl, form as seawater evaporates.

Figure 1. Salt harvesting at Lake Grassmere , New Zealand. The pink colour comes from a salt-loving (halophile) algae.
What is happening? Does the seawater contain particles of sodium chloride (NaCl)? No. Seawater contains sodium ions (Na+) and chloride ions (Cl–), each surrounded by water molecules. The water molecules act as a shield, muffling the charges on the Na+ and Cl- ions so they don't attract each other. As the seawater evaporates there is no longer enough water to keep the ions apart and their opposite electric charges allow them to get close and form solid sodium chloride.
What about the reverse process, dissolving sodium chloride in some water? It is obvious that we can't add salt indefinitely. Did you know that the amount that can dissolve differs from substance to substance? The maximum amount of a substance that can be dissolved in a given volume of water is called the solubility and is a fundamental property of the substance. For sodium chloride a surprising 360 g will dissolve per kg of freshwater at room temperature; for sodium fluoride, a close relative, the value is only 41 g. Calcium carbonate is even less soluble: only about 0.01 g (10 mg) will dissolve will dissolve under the same conditions. (Spoiler alert: Post 12 will discuss the different forms of calcium carbonate that have different solubilities and will go over the critical difference this will make under ocean acidification).
A solution that contains the maximum possible amount of a substance is described as being saturated. The corollary of this is that if you have a saturated solution and then add more of one or both of the component ions in the salt, (i.e. if you exceed the solubility and have too many ions in solution), then the substance will begin to precipitate until the excess has been removed and the solution is back to being saturated.
However, as well as being saturated, a solution can be supersaturated. This means the solution contains more ions that can combine to form a salt than is 'theoretically' possible. Plainly it is possible, since the surface seawater is supersaturated with CaCO3. Remember, the solubility of CaCO3 in freshwater is only 10 mg per kg. In comparison, the concentration of Ca2+ (the 3rd most abundant cation in seawater, after sodium and magnesium) is 412 mg (!) per kg of seawater and the surface concentration of bicarbonate HCO3– and carbonate CO3– (post 5 will explain how bicarbonate and carbonate can interconvert) is 114 mg per kg of seawater. These concentrations are much higher than we would predict from simple solubility calculations alone. This means it takes very little effort to precipitate CaCO3 from seawater and the making of shells should be easy.
In the next post we remind readers about pH and discuss the recent changes to the surface seawater.
Written by Doug Mackie, Christina McGraw , and Keith Hunter . This post is number 3 in a series about ocean acidification. Other posts: Introduction , 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, Summary 1 of 2, Summary 2 of 2.
Posted by Doug Mackie on Friday, 8 July, 2011
 |
The Skeptical Science website by Skeptical Science is licensed under a Creative Commons Attribution 3.0 Unported License. |