To tweet or not to tweet at Donald Trump? That was the question!
Posted on 8 March 2017 by BaerbelW, John Mason
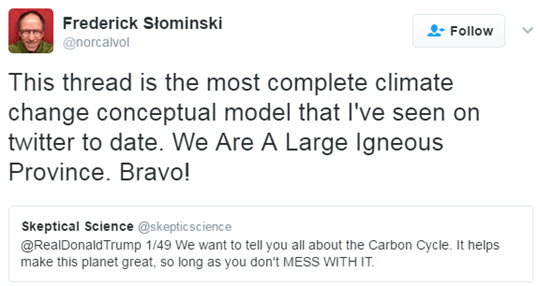
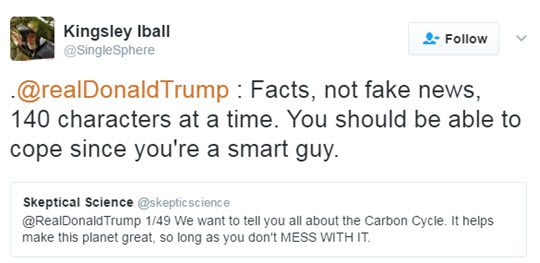
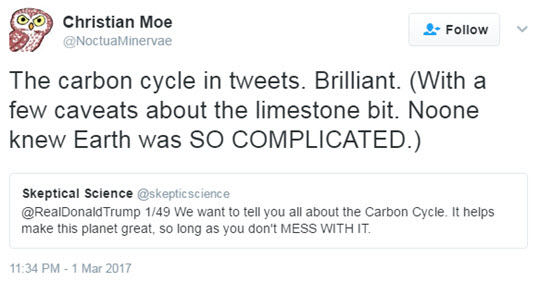
Knowing Twitter to be the prefered means of communication for the current POTUS and that he “may” have a thing or two to learn about climate science, John Mason recently set out to explain the carbon cycle in a series of 49 tweets in a language we hoped Donald Trump would be able to grasp.
As John explained: “I often wonder if a lot of climate change communication follows formats that may be unattractive to some people. Lengthy posts complete with explanatory graphics are appreciated by many, but others simply may not have the time to work through them for all sorts of reasons. Yet, this should not exclude them from accessing information. So regardless of whether Trump read the tweets or not, I wanted to proceed with this as an experiment in making climate communication available to a wider demographic. The simpler the framing of information, the more quickly it may be scanned and absorbed. I picked a fairly complex aspect of planetary science - Earth’s Carbon Cycle - and set out to simplify it whilst keeping it consistent with what the science says.
So, on February 28, the tweets started to go out on Twitter in a little tweet storm:
A good two hours later the final tweets were sent:
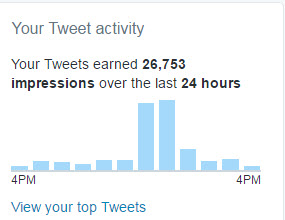
 This little storm showed up in our Twitter stats almost immediately where it more than doubled the impressions compared to a typical day where we get around 12,000:
This little storm showed up in our Twitter stats almost immediately where it more than doubled the impressions compared to a typical day where we get around 12,000:
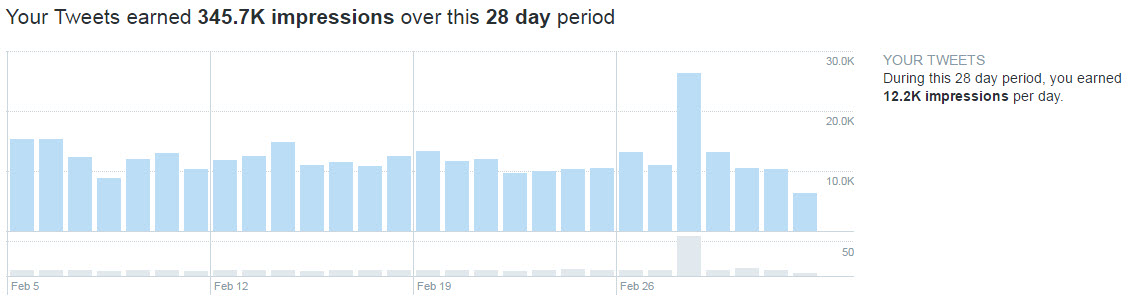
The peak is even more pronounced if we look at the past 28 days:
We of course don’t know if Donald Trump read - or even noticed - any of these tweets and if he did, whether he understood them. But, from some obviously not representative sample of comments we know that at least some people DID learn from this experimental blog post. Here are a few examples:
From Twitter:
 On John Mason’s Facebook page:
On John Mason’s Facebook page:
AO - Yes! I like bite size information. Even though I've got a keen interest in the topic, I often feel overwhelmed by pages long worthy articles or I simply haven't got the time to read them.
AT - Same here.
I use social media on the whole to escape the news and the mundanity of life and to av a larf like innit.
So I also tend to avoid lengthy serious articles always intending to read them later and never do.
JS - Don't recall us being compared to a dinosaur killing volcano before...good work ! A few words have too many syllables for the intended recipient...but the language and tone is awesome :-) :-) :-) sharing with a few youngstars' teachers...maybe it will be more appealing than the usual offerings.
CD - Brilliant broken down like that for climate ignoramuses like me and Trumpton...though the comments from the must correct brigade left me cold nit picking over details...the fact that mans contribution to the destruction of our planet = a catastrophic planet destroying volcanic eruption was a visual image even I, a science idiot understood! I think Trump would have lost interest by the time the real info started to be explained though....he has the attention span of a squashed gnat by all accounts…
What about limestone?
The blog post hadn't been online for long, when a little discussion about some details regarding limestone (tweet2 23 & 24) did perhaps miss the point of the post and the tweets a little bit:
“23 & 24 so WRONG! Calcification a SOURCE not sink of CO2. See Equation 1 of OA not OK (link left side bar). COMPLEX - some buts and ifs.”
John Mason provided an explanation:
“In terms of total carbon though (which is the subject here), half the carbon from bicarbonate swishing around in the sea gets locked away as calcium carbonate. Hence the specific description of limestone as a CARBON sink, not a CO2 sink. Every description of the slow carbon cycle mentions the same. Carbon locked up in limestone is mostly out of the way, unless it gets cooked or weathered - and for a lot of limestone that's only a minor, localised process.”
John also notes: “according to the IPCC, the glossary definition of a "sink" is:
Any process, activity or mechanism that removes a greenhouse gas (GHG), an aerosol or a precursor of a GHG or aerosol from the atmosphere. {WGI, II, III}
The overall equation of the process of basalt weathering and carbonate precipitation is often expressed as follows:
CO2 + CaSiO3 = CaCO3 + SiO2
That satisfies the IPCC definition - even if integral parts of what is a multi-stage process do not! However the same definition is satisfied by photosynthesis, even though it is seasonal in nature and balanced out by other parts of the fast carbon cycle that return biogenic CO2 to the atmosphere.
However, it is important that people understand that limestone stores vast amounts of carbon out of harm’s way. Perhaps a more Trumpish description of limestone would be a carbon TRAP. Stuck right in there. Loser.
This "limestone issue" also made it into a tweet:
Such teething troubles are in any case to be expected when attempting to distill complex processes into simple words and we intend to continue with this interesting experiment. We’ll be exploring some other aspects of the Earth-Sun-climate system, using the same format, in the coming weeks and any constructive feedback is very much appreciated.































 Arguments
Arguments






























Dancing around a definition like this and introducing something not previously discussed (basalt) is EXACTLY what deniers do. SHAME!
[JH] The use of all-caps constitutes shouting and is prohibited by the SkS Comments Policy.
Amended: Because the commenter is posting a mock tweet, they will be allowed.
Calcification (formation limestone) removes 2 bicarbonate & releases 1 CO2 in ocean. Not realistic to imply bicarbonate precursor to a GHG
Weathering basalt so slow it is OBFUSCATION to mention. Weathering limestone releases bicarbonate; insignificant to CC on human time scale
[JH] All-caps snipped.
Amended: Because the commenter is posting a mock tweet, they will be allowed.
Doug, the Slow Carbon Cycle is called just that for fairly obvious reasons. Yes it does mostly operate on geological timescales, but there is evidence for periods of highly enhanced weathering. These are considered to be preserved in the Sr isotope record e.g. in the run-up to the Hirnantian glaciation/extinction (ref below). The complex process of Ca-bearing silicate weathering by atmospheric CO2 dissolved in rainwater through to deposition of carbonate sediments is an overall remover of carbon, locking it up within limestone. Since Ca-silicate weathering and limestone deposition are both ongoing processes worldwide, there is a continuous flux of carbon from the air into the lithosphere. The quantity of carbon thus stored away in limestones is phenomenal. You will note that I talk about carbon as opposed to carbon-bearing species, as regardless of your points it cannot be denied that the process begins with carbon in the form of CO2 and its interactions with water and calc-silicates:
2CO2 + 3H2O + CaSiO3 = 2HCO3 – + Ca2+ + H4SiO4
and ends with calcium carbonate deposition:
2HCO3 – + Ca2+ = CO2 + H2O + CaCO3
2 moles of carbon (as CO2) at the start; 1 mole returned as CO2 at the end, 1 mole locked away in calcium carbonate. Overall, that whole bit of the Slow Carbon Cycle results in a net loss of atmospheric CO2.
That's the process going one way. But perturbations of the Slow Carbon Cycle in the opposite sense can occasionally be much more rapid - the Siberian Traps magmas cooking a thick oil/coal-rich sedimentary basin sequence being one example. Mankind's burning of the fossil fuels is another. The point is that the Slow Carbon Cycle both stores and releases carbon continuously, but great big carbon burps can occasionally occur, for which the consequences tend not to be pretty!
Ref: Young, S.A., Saltzman, M.R., Foland, K.A., Linder, J.S. and Kump, L.R. (2009): A major drop in seawater 87Sr/86Sr during the Middle Ordovician (Darriwilian): Links to volcanism and climate? Geology, October 2009, v. 37, p. 951-954.
I hang around Skeptical Science and learned a lot from the pill size tweets, so I think this is a really good idea, although I seldom use tweeter.
John Mason@4,
You know better than me that Slow Carbon Cycle, no matter how far back we can look into the past has never had the sequestering rate (maily silicate weathering) nearly as fast as to make any difference in the current antropogenic release, the release comparable to largest LIPs on record.
Therefore, there is no point talking about silicate weathering in th econtext of carbon cycle balancing we are facing in antropocene: it will never help us. All CC perturbtions in geo-history were fast releases (LIPs) and slow drawdown (increased weathering). Increased drawdowns (changes in weathering rates) never matched the changes in releases (LIPs), weathering were always slower by a factor of 100.
Slow carbon irrelevant to CC & OA. Deceptive to jiggle ‘sink’ definition & talk basalt, original tweet limestone. Why you DENIALIST TACTICS?
[JH] All-caps snipped.
Amended: Because the commenter is posting a mock tweet, they will be allowed.
Chris - yes I think you'll find I've made that abundantly clear. LIPs and human combustion of fossil fuels are rare instances of very rapid perturbations of the slow carbon cycle. Even periods of vastly enhanced weathering of mafic rocks are slow compared to a) the Siberian Traps or b) anthropogenic fossil fuel combustion, although they can be significant nevertheless. Ref. the "weathering goes crazy" tweet and the ones giving figures for the Traps and manmade emissions. The point being that the slow carbon cycle goes along fine and dandy - unless it gets messed with by something of a dramatic nature.
Doug, fossil fuel combustion is a process that yanks gigatons of carbon out of the slow carbon cycle. I struggle to see why you view that as an irrelevance.
Doug Mackie, chemistry tends to be a mystery to me, so I am asking for you to clarrify some points. To begin with, according to David Archer, after the initial uptake of a pulse of CO2 by the ocean, there is a period of about 5000 years in which the "reaction with CaCO3", which significantly draws down the atmspheric CO2:
While 5000 years is a long time in historical terms, it is still a relevant human time scale.
My understanding is that the same process will return the ocean pH to approximately preindustrial levels.
Further, as I understand it, the chemical reaction involved is:
CO2 + CaCO3 + H2O <-> 2HCO3- + Ca2+
Looking at the equilibrium chart for carbon species in the ocean, it appears to me that the reaction draws down CO2 from the atmosphere be drawing down the pool of aqueous CO2/H2CO3, with the additional effect of shifting the equilibrium balance towards reduced CO2/H2CO3 relative to HCO3- due to the shift in pH:
While I am unsure that that is the precise mechanism, it appears this reaction is sufficiently useful at drawing down CO2 that it has been proposed as a method to reduce atmospheric CO2 artificially.
As I said, chemistry tends to be a mystery to me, so I may well have got one or more points wrong on this. Could you explain to me where I am in error, preferably in a comment longer than a tweet and without all caps.
You are jerks to use CAPS in tweets but not allow in comments. I no longer wish to be assoc with sks. Please remove OA not OK series by me
[GT]
Doug. The policy on SkS restricts ALLCAPS as shouting. However you have the option of using bold as an alternative method which says 'this is a bit more important than usual but I don't want to yell at you'
Seemingly Twitter hasn't caught up yet.
Doug... Please read the comments policy. This has been a long standing policy here on the website, going back as far as I can remember. And a quick look and I don't see anywhere all caps is used for SkS tweets.
Rob Honeycutt @12, at a quick perusal, 7 of the 49 tweets include all caps (8,11,14,26,35,36, and 49). That, however, is irrelevant. The tweets are not comments at SkS, so the moderation policy at SkS does not apply. Further, it is not apparent to me that you can use any textual method to increase emphasis on twitter other than by all caps (but I don't tweet and may be entirely wrong about that).
I will go further. I have a serious objection to twitter in that the short character count per message makes any serious discussion impossible. Even the creative method of using a 49 tweet sequence leaves you using short sentences that do not convey any reasonable level of subtlety. Doug Mackie in his responses here appears to be treating this comments section as a twitter account. His responses have been too short to make a reasonable assessment of his objections beyond the mere fact that he objects. They have been rude, and inflammatory to boot.
On top of that, I struggle to see his objection to the content of the tweets. Atmospheric CO2 would be astronomically higher if the carbon locked up in carbonates were free to bond with oxygen in the atmosphere. That is what the tweet he objects to says. It seems incontrovertible. Even on the narrower point of whether adding calcium carbonate to the the ocean by weathering will draw down CO2, he appears to be arguing against the science. Where I discussing this with a denier, I would probably attribute the nature of his posts (as long on bombast as they are short in length) to that fact. As it is, I remain perplexed both as to the nature of his objections, and they way he has argued his point, including his final denunciation.
As far as the IPCC definition is concerned, and it's been there for a while, the formation of limestone adds carbon dioxide to the atmosphere. So it cannot be considered a sink.
On the other hand, weathering, the dissolution of limestone, does remove carbon dioxide from the atmosphere. This process satisfies the IPCC definition of a sink.
It's confusing as hell to non-experts given that the ultimate fate of much of that carbon, over geological timescales, is to end up in limestone sediments, but we shouldn't attempt to reinterpret what constitutes a sink.
I agree it's confusing, Rob!
As a geologist I view the whole thing as a pathway between weathering and precipitation. Importantly, as shown by the following weathering equations:
Silicate weathering:
2CO2 + 3H2O + CaSiO3 = Ca2++ 2HCO3– + H4SiO4
Carbonate weathering:
CO2 + H2O + CaCO3 = Ca2++ 2HCO3–
the weathering of limestone only involves one mole of atmospheric CO2 whereas silicate weathering involves two. In both cases though, when it comes to the reprecipitation part, a mole of carbon goes into the reservoir and a mole of CO2 is released. So only in silicate weathering is there a net removal of atmospheric CO2; limestone weathering is net neutral.
Doug, I agree that with specific reference to limestone, carbon reservoir would have been better. Amazingly, it just fits! I've changed it now :)
My research background is in mineralisation (specifically metallogenesis and supergene alteration). Especially in the latter, we see various geochemical pathways from one mineral to another (e.g. from galena to cerussite) with intermediate and relatively metastable reservoir species along the way.
In the case of the weathering of Ca-bearing silicates, I can plead that I was trying to simplify things as far as I could – that was the whole idea of the exercise. Sure, not every ion of basalt-derived HCO3- ends up in limestone – any one ion might or might not see that outcome, but the whole process can be viewed as a pathway in simplistic terms.
A longer-winded version would have been to say that the weathering of Ca-silicate bearing rocks draws down 2 moles of CO2 for each mole of Ca-silicate consumed and adds it to the oceanic HCO3- reservoir. Limestone formation takes two moles of carbon from that reservoir, combining one into calcium carbonate, and releasing one as carbon dioxide. Difficult in 140 characters.
Yes I absolutely agree these are parts of the slow carbon cycle. However, the slow carbon cycle is not, as you say, “utterly irrelevant to CC “, unless you only mean ongoing CC, where we've thrown a massive spanner into the works of another part of the SCC. The post does not touch upon OA – if it did it would have had to be a great deal longer.
The part of the slow carbon cycle that involves weathering is important in climate change. Not necessarily right at the moment (although some geo-engineering types are enthusiastic about it), but very much so with respect to climate change episodes in the deep past, the understanding of which is helpful to our knowledge of the climate of today.
There is a considerable slab of the literature devoted to the weathering process through geological time and especially to instances of enhanced silicate weathering which may be of sufficient extent and rate as to draw down enough atmospheric CO2 to bring about episodic cooling e.g. see the Young et al paper cited above. There is likewise a lot of debate as to the role of weathering in the Cryogenian (plenty via Google Scholar).
** Climate change refers to a change in the state of the climate that can
be identified (e.g. using statistical tests) by changes in the mean and/
or the variability of its properties, and that persists for an extended
period, typically decades or longer. Climate change may be due
to natural internal processes or external forcings, or to persistent
anthropogenic changes in the composition of the atmosphere or in
land use.
Note that UNFCCC, in its Article 1, defines “climate change” as “a
change of climate which is attributed directly or indirectly to human
activity that alters the composition of the global atmosphere and
which is in addition to natural climate variability observed over
comparable time periods”. The UNFCCC thus makes a distinction
between “climate change” attributable to human activities altering
the atmospheric composition, and “climate variability” attributable
to natural causes.
From the IPCC glossary. However, the top definition is the one used widely in the literature e.g. put "climate change Cenozoic" into Scholar and see what comes up!
@Doug Mackie: Re the "all-caps" brouhaha — At the time I struck your all-caps words, I did not realize that your comments were meant to be mock tweets. I apologize.