 Arguments
Arguments
 Software
Software
 Resources
Comments
Resources
Comments
 The Consensus Project
The Consensus Project
 Translations
Translations
 About
Support
About
Support


Latest Posts
- EGU2025 - Presentation about our translation activities
- Skeptical Science New Research for Week #17 2025
- Inside my quest for a climate-friendly bank
- Sabin 33 #25 - Are wind projects hurting farmers and rural communities?
- A worse-than-current-policy world?
- 2025 SkS Weekly Climate Change & Global Warming News Roundup #16
- Fact brief - Is climate change a net benefit for society?
- Skeptical Science New Research for Week #16 2025
- Climate Adam: Climate Scientist Reacts to Elon Musk
- Sabin 33 #24 - Is wind power too expensive?
- EGU2025 - Picking and chosing sessions to attend on site in Vienna
- 2025 SkS Weekly Climate Change & Global Warming News Roundup #15
- Fact brief - Is the sun responsible for global warming?
- Skeptical Science New Research for Week #15 2025
- Renewables allow us to pay less, not twice
- Sabin 33 #23 - How much land is used for wind turbines?
- Our MOOC Denial101x has run its course
- 2025 SkS Weekly Climate Change & Global Warming News Roundup #14
- Fact brief - Is Mars warming?
- Skeptical Science New Research for Week #14 2025
- Two-part webinar about the scientific consensus on human-caused global warming
- Sabin 33 #22 - How does waste from wind turbines compare to waste from fossil fuel use?
- Clean energy generates major economic benefits, especially in red states
- 2025 SkS Weekly Climate Change & Global Warming News Roundup #13
- Skeptical Science New Research for Week #13 2025
- Climate skeptics have new favorite graph; it shows the opposite of what they claim
- Sabin 33 #21 - How does production of wind turbine components compare with burning fossil fuels?
- China will need 10,000GW of wind and solar by 2060
- 2025 SkS Weekly Climate Change & Global Warming News Roundup #12
- Skeptical Science New Research for Week #12 2025
Archived Rebuttal
This is the archived Intermediate rebuttal to the climate myth "CO2 emissions do not correlate with CO2 concentration". Click here to view the latest rebuttal.
What the science says...
|
When CO2 emissions are compared directly to CO2 levels, there is a strong correlation in the long term trends. This is independently confirmed by carbon isotopes which find the falling ratio of C13/C12 correlates well with fo |
To directly compare CO2 emissions to atmospheric CO2 levels, both sets of data can be converted to gigatonnes of CO2. The CO2 emissions data is typically expressed in gigatonnes carbon (GtC). One gigatonne is equal to one billion tonnes. This means they've only included the carbon element of the carbon dioxide molecule. The atomic mass of carbon is 12, while the atomic mass of CO2 is 44. Therefore, to convert from gigatonnes carbon to gigatonnes of carbon dioxide, you simply multiply 44 over 12. In other words, 1 gigatonne of carbon equals 3.67 gigatonnes of carbon dioxide.
Atmospheric CO2 levels are expressed in parts per million by volume (ppm). To convert from ppm to gigatonne of carbon, the conversion tables of the Carbon Dioxide Information Analysis Center advise that 1 part per million of atmospheric CO2 is equivalent to 2.13 Gigatonnes Carbon. Using our 44 over 12 rule, this means 1ppm = 7.8 Gigatonnes of Carbon Dioxide in the atmosphere.
[Note that the conversion is different for Gigatonnes of Carbon Dioxide emissions, because natural sinks (ocean and biosphere) absorb approximately 55% of human emissions, so the "airborne fraction" added to the atmosphere is about 45%. This means 1ppm = 17.3 Gigatonnes of Carbon Dioxide emissions.]
The two time series can both be plotted together expressed as gigatonnes of carbon dioxide:
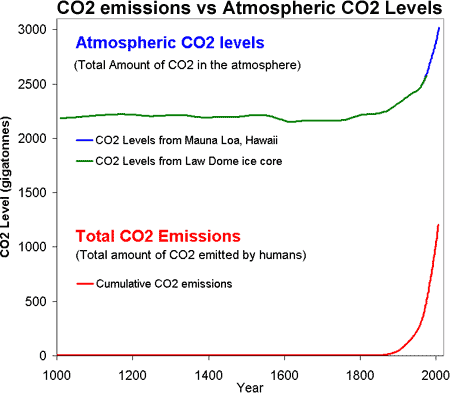
Figure 1: CO2 levels (Green Line - Law Dome, East Antarctica and Blue line - Mauna Loa, Hawaii) and Cumulative CO2 emissions in gigatonnes of CO2 (Red Line - CDIAC).
So putting it all together, Figure 1 is a plot of the total amount of CO2 in the atmosphere (top) versus the total amount of CO2 humans have emitted into the atmosphere (bottom). Several features jump out. Firstly, the similar shape of the curves (dare I say hockey stick shaped). We have correlation but do we have causality?
It isn't too much of a stretch to imagine the amount of CO2 we put into the atmosphere might have a causality link with the amount of CO2 that remains in the atmosphere. Nevertheless, further confirmation comes by analysing the types of CO2 found in the air. The carbon atom has several different isotopes (eg - different number of neutrons). Carbon 12 has 6 neutrons, carbon 13 has 7 neutrons. Plants have a lower C13/C12 ratio than in the atmosphere. If rising atmospheric CO2 comes fossil fuels, the C13/C12 should be falling. Indeed this is what is occuring (Ghosh 2003) and the trend correlates with the trend in global emissions.
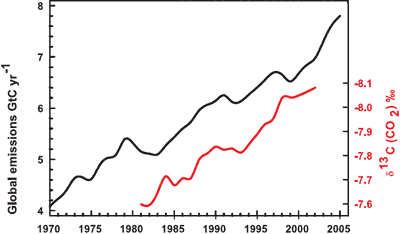
Figure 3: Annual global CO2 emissions from fossil fuel burning and cement manufacture in GtC yr–1 (black), annual averages of the 13C/12C ratio measured in atmospheric CO2 at Mauna Loa from 1981 to 2002 (red). (IPCC AR4)
Updated on 2020-02-19 by John Cook.
THE ESCALATOR

(free to republish)
























































