Arguments
Arguments
 Software
Software
 Resources
Comments
Resources
Comments
 The Consensus Project
The Consensus Project
 Translations
Translations
 About
Support
About
Support


Latest Posts
- 2025 SkS Weekly Climate Change & Global Warming News Roundup #14
- Fact brief - Is Mars warming?
- Skeptical Science New Research for Week #14 2025
- Two-part webinar about the scientific consensus on human-caused global warming
- Sabin 33 #22 - How does waste from wind turbines compare to waste from fossil fuel use?
- Clean energy generates major economic benefits, especially in red states
- 2025 SkS Weekly Climate Change & Global Warming News Roundup #13
- Skeptical Science New Research for Week #13 2025
- Climate skeptics have new favorite graph; it shows the opposite of what they claim
- Sabin 33 #21 - How does production of wind turbine components compare with burning fossil fuels?
- China will need 10,000GW of wind and solar by 2060
- 2025 SkS Weekly Climate Change & Global Warming News Roundup #12
- Skeptical Science New Research for Week #12 2025
- Climate Fresk - a neat way to make the complexity of climate change less puzzling
- Sabin 33 #20 - Is offshore wind development harmful to whales and other marine life?
- Do Americans really want urban sprawl?
- 2025 SkS Weekly Climate Change & Global Warming News Roundup #11
- Fact brief - Is waste heat from industrial activity the reason the planet is warming?
- Skeptical Science New Research for Week #11 2025
- Visualizing daily global temperatures
- Sabin 33 #19 - Are wind turbines a major threat to wildlife?
- The National Hurricane Center set an all-time record for forecast accuracy in 2024
- 2025 SkS Weekly Climate Change & Global Warming News Roundup #10
- Fact brief - Is Greenland losing land ice?
- The Cranky Uncle game can now be played in 16 languages!
- Skeptical Science New Research for Week #10 2025
- Climate Adam: Protecting our Planet from President Trump
- Sabin 33 #18 - Can shadow flicker from wind turbines trigger seizures in people with epilepsy?
- Cuts to U.S. weather and climate research could put public safety at risk
- 2025 SkS Weekly Climate Change & Global Warming News Roundup #09
Archived Rebuttal
This is the archived Basic rebuttal to the climate myth "CO2 increase is natural, not human-caused". Click here to view the latest rebuttal.
What the science says...
At a glance
Do you believe in the Tooth Fairy? A mysterious entity that turns up when people are sleeping, to remove unwanted things and replace them with something nicer? Because, you see, Murry Salby's ramblings require a Tooth Fairy to help him out. Let's take a look.
Murry Salby was a briefly popular character in the circus that is organised climate science denial, with the same few dozen names cropping up repeatedly in books, conferences, speaking tours and so on. If Salby was right, the army of scientists that have worked on the carbon cycle over many decades must have missed something glaringly obvious. They have not.
The fast part of the carbon cycle is represented by the annual near-symmetrical 'wiggle' on graphs of CO2 concentration. The peaks and troughs of the wiggle pretty much cancel one another out. This is unsurprising when one considers their source - the living world and particularly plants.
Plants take in CO2 when they are in the growing season - so the concentration falls by a few parts per million (ppm), hence the troughs. In the depths of winter, many plants die or enter dormancy and the opposite happens, hence the peaks. The sizes of the peaks and troughs both fluctuate together depending on things like certain natural climate cycles. For example, in the well-known El Nino Southern Oscillation, vegetation takes up more CO2 during La Nina.
There's also the slow carbon cycle that operates over geological time-spans of thousands to millions of years. We have a range of tools with which to interrogate the record of the slow carbon cycle. An obvious one is air bubbles trapped in ancient glacial ice and sampled from ice-cores. Over pre-industrial Holocene times (11,700 years ago-recent), CO2 concentrations show little variation - an erratic 20 ppm (or about 7%) increase over all that time. That suggests the net natural CO2 flux was small: what the planet was putting into the atmosphere was largely taken back out. Going back further, into the glacial-interglacial cycles, we see that CO2 fell to less than 200 ppm in the ice-ages and in the milder interglacials it rose to about 280 ppm. That was the case from at least a million years ago.
Now, all of a sudden, CO2 has shot up to around 420 ppm since the late 19th Century. It's gone up 50% in less than 150 years. What's the big difference about the world now and the one over the previous million years? The answer is the intensification of the industrial era, post-1950. Back then we were emitting 6 billion tonnes of CO2 per annum. That figure has now risen to 44.25 billion tonnes a year - it's gone up more than sevenfold.
If you still insist the recent CO2 increase is not due to human activity, you need to make that 44.25 billion tons of emissions per annum (and rising) magically disappear somehow. That's where the Tooth Fairy has to come to your aid – and as any rational person knows, it doesn't in fact exist.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
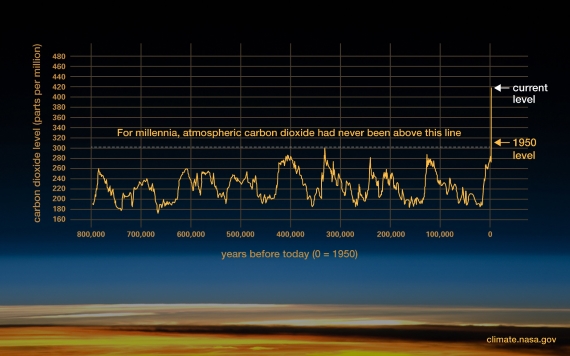
Atmospheric CO2 has increased by more than 140 parts per million (or 50% if you prefer) since the Industrial Revolution when humans began burning fossil fuels like coal and oil in earnest. Human industrial activity has increased atmospheric CO2 to levels not seen for at least 800,000 years (fig. 1).
Figure 1: Atmospheric CO2 concentrations in parts per million (ppm). Levels have peaked regularly throughout recent geological time but we’ve seen a steep increase of 140 PPM since the industrial revolution. Graphic: Climate.NASA
Carbon moves between the atmosphere, oceans, biosphere and solid Earth in various processes that, in combination, are called Earth's Carbon Cycle. The Carbon Cycle has operated in one form or another since Earth came into existence. It has mostly been stable, allowing advanced life to develop and flourish here.
Salby was well-known for insisting humans are not the cause of the recent 50% CO2 increase, citing 'natural variation'. Technically, Salby's arguments involve the fallacy of 'slothful induction'. That means ignoring relevant information in order to come to a conclusion. Put more simply, where on Earth does Salby think our 44,000 million tons of CO2 emissions per year (as of 2019) actually end up? Is there some kind of secret galactic plughole they vanish down, never to return?
Slothful induction involves blindly ignoring multiple sources of evidence that burning fossil fuels has increased CO2 levels in Earth's atmosphere. We know 'natural variation' is not the source of growing CO2 levels in the atmosphere because land and ocean CO2 storage has increased. How do we know?
Most of Earth’s carbon is stored in the rocks making up the solid Earth (Fischer et al. 2020). The rest is in the ocean, the atmosphere and the biosphere. Oceans form an important store, so if all the recent atmospheric CO2 increase were 'natural' - and that would involve fossil fuels having never been exploited - the oceans would be one obvious source. But we know the CO2 increase is not coming from the oceans because the pH of the oceans is dropping. The oceans are instead absorbing increased CO2 and that process leaves a fingerprint, known as ocean acidification.
Ocean acidification works thus: when CO2 is dissolved into sea water, it binds with a water molecule to form a molecule of carbonic acid (H2CO3). The acidifying effect is due to 95% of that carbonic acid turning into bicarbonate ions [HCO3-]. Every time a carbonic acid molecule splits into bicarbonate, a hydrogen ion (H+) is also liberated. The more CO2 is absorbed into the oceans, the more the above process goes on and the more H+ ions are produced, so that the ocean pH decreases. Falling oceanic pH thus shows that our oceans are absorbing more carbon than they are releasing.
Isotopic Signature Shows Increased Fossil Fuels Emissions in Atmosphere
Another smoking gun is that carbon isotope chemistry points squarely at fossil fuels as the source of CO2 emissions. Carbon is composed of three different isotopes: carbon-12, carbon-13 and carbon-14. Carbon-12 is by far the most common (98.9%), while carbon-13 makes up most of the rest. Carbon-14 is in contrast only a tiny fraction of the total.
All photosynthetic plants preferentially process the lighter carbon isotope, carbon-12. This is because in some of the chemical reactions involved in photosynthesis, the energetics favour light carbon over carbon-13. As a consequence, plant tissues have relatively low carbon-13 to 12 ratios, compared to the bulk Earth.
Animals eat plants or each other, ensuring that the low carbon isotope ratio spreads out through food-chains. Now, as we know, fossil fuels are derived from ancient organic matter consisting of plant and animal-remains. It follows that fossil fuels carry that same biogenic carbon-13 to 12 ratio. So if we dig up and set fire to those fossil fuels, that isotopic signature is passed on into the resultant CO2. In that way, it transferred into the atmosphere.
Reconstructions of atmospheric carbon isotope ratios are made from various geological sources. Common examples are ice cores and marine carbonate sediments of known age. Such records have determined that the carbon-13 to 12 ratios in the atmosphere are currently the lowest in the last 10,000 years. In addition, the carbon-13 to 12 ratios begin to decline dramatically just as CO2 started to increase after around 1850 AD (fig. 2). This is exactly what we would expect if the increased CO2 is due to fossil fuel burning.
In addition, these isotopic observations confirm that the increase in atmospheric CO2 comes from plant-based carbon, not from the oceans or volcanoes (Quay et al. 1992). Magmatic CO2 has a near-bulk Earth isotopic composition.
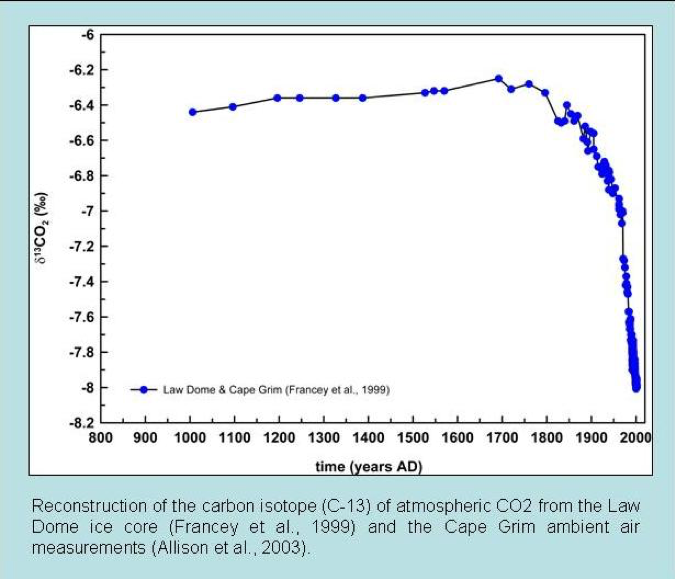
Figure 2: Ice core carbon isotope measurements of atmospheric CO2 over the past twelve centuries (Francey et al. 1999).
Some argue that the biogenic carbon-13 ratio isn't unique to fossil fuels. They're missing something else here and that's where carbon-14 puts in a guest-appearance. Due to the very short half-life of carbon-14 - just a few thousand years - ancient biogenic carbon, millions of years old, contains hardly any of it. But as we burn more and more gigatons of fossil fuels, that ancient biogenic signature is diluting the tiny amount of carbon-14 up there in the atmosphere. This is not new science either, it's something we've known for over half a century (Revelle & Suess 1957), and there have been many studies confirming these results, for example, Levin & Hesshaimer (2000).
Fossil fuel burning is the key reason for the recent 50% leap in atmospheric CO2 levels. More evidence, if you need it, is presented in the Intermediate rebuttal. But the conclusion should already be obvious. Due to our fossil fuel-burning activities, CO2 levels have surged upwards. Science-deniers try to sweep this glaring fact under the carpet, but their arguments simply don't hold water.
Updated on 2023-12-09 by Ken Rice.
THE ESCALATOR

(free to republish)