How do we know more CO2 is causing warming?
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
|
 Advanced
Advanced
| ||||
|
An enhanced greenhouse effect from CO2 has been confirmed by multiple lines of empirical evidence. |
|||||||
Climate Myth...
Increasing CO2 has little to no effect
"While major green house gas H2O substantially warms the Earth, minor green house gases such as CO2 have little effect.... The 6-fold increase in hydrocarbon use since 1940 has had no noticeable effect on atmospheric temperature ... " (Environmental Effects of Increased Atmospheric Carbon Dioxide)
At-a-glance
To make a statement like, "minor greenhouse gases such as CO2 have little effect", is to ignore 160 years of science history. So let's look at who figured out the heat-trapping properties of carbon dioxide and when.
Experiments involving various gas mixtures had demonstrated the heat-trapping properties of water vapour, CO2 and methane in the 1850s. But those effects were yet to be quantified - there were no meaningful numbers. It was to be another 40 years before that happened.
Swedish scientist Svante Arrhenius (1859-1927) was the person who crunched the numbers. The results were presented in a remarkable paper, "On the Influence of Carbonic Acid in the Air upon the Temperature of the Ground", in 1896.
The many calculations in the 1896 paper include estimates of the amounts of CO2 increase or decrease required to drive the climate into a different state. One example used was the Hothouse climate of the Cenozoic, around 50 million years ago. Another was the glaciations of the last few hundred millennia.
To get a temperature rise of 8-9°C in the Arctic, Arrhenius calculated that CO2 levels would have to increase by 2.5 to 3 times 1890s levels. To lower the temperature 4–5°C to return to glacial conditions, he calculated a drop in CO2 was needed of 0.62-0.55 times 1890s levels.
We know CO2 levels in the 1890s from ice-core data. They were around 295 ppm. Let's do the sums. A reduction factor of 0.55 to 0.62 on 295 ppm gives 162.2-183.9 ppm. Modern ice-core measurements representing the past 800,000 years show that in glacial periods, CO2 levels fell to 170-180 ppm.
What we now know due to additional research since 1896 when Arrhenius worked on this, is that CO2 was an essential 'amplifying feedback'. That means changes triggered by long term, cyclic variations in Earth's orbit cause warming or cooling and CO2 release or entrapment in turn. Those changes in CO2 levels affected the strength of Earth's greenhouse effect. Changes in the strength of the greenhouse effect then completed the job of pushing conditions from interglacial to glacial - or vice-versa.
Arrhenius also made an important point regarding water vapour: "From observations made during balloon voyages, we know also that the distribution of the aqueous vapour may be very irregular, and different from the ideal mean distribution." This statement holds true today: water vapour is a greenhouse gas but because water exists in gas, liquid and solid forms in the atmosphere, it is continually cycling in and out of the air. It is distributed in a highly uneven fashion and is uncommon in the upper atmosphere. That's where it differs from CO2.
Once CO2 is up there, it's up there for a long time. As a consequence it has a pretty even distribution: 'well-mixed' is the term. As Arrhenius quantified all that time ago, once it's up there it constantly absorbs and re-radiates heat in all directions. That's why dumping 44 billion tons of it into our atmosphere in just one year (2019 - IPCC Sixth Assessment Report 2022) is a really bad idea.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
Good scientific theories are said to have ‘predictive power’. In other words, armed only with a theory, we should be able to make predictions about a subject. If the theory’s any good, the predictions will come true.
Here’s an example: when the Periodic Table of the chemical elements was proposed in 1869, many elements were yet to be discovered. Using the theory behind the Periodic Table, the Russian chemist Dmitri Mendeleev was able to predict the properties of germanium, gallium and scandium prior to their discovery in 1886, 1875 and 1879 respectively. His predictions were found to be correct.
The effect on Earth's greenhouse effect of adding man-made CO2 is predicted in the theory of greenhouse gases. This theory was first proposed by Swedish scientist Svante Arrhenius in 1896, based on earlier work by Fourier, Foote and Tyndall. Many scientists have refined the theory since Arrhenius published his work in 1896. Nearly all have reached the same conclusion: if we increase the amount of greenhouse gases in the atmosphere, the Earth will warm up.
Where there is less agreement is with respect to the exact amount of warming. This issue is called 'climate sensitivity', the amount the temperatures will increase if CO2 is doubled from pre-industrial levels. Climate models have predicted the least temperature rise would be on average 1.65°C (2.97°F) , but upper estimates vary a lot, averaging 5.2°C (9.36°F). Current best estimates are for a rise of around 3°C (5.4°F), with a likely maximum of 4.5°C (8.1°F). A key reason for this range of outcomes is because of the large number of potential climate feedbacks and their variable interactions with one another. Put simply, some are much better understood than others.
What Goes Down…
The greenhouse effect works like this: Energy arrives from the sun in the form of visible light and ultraviolet radiation. The Earth then emits some of this energy as infrared radiation. Greenhouse gases in the atmosphere 'capture' some of this heat, then re-emit it in all directions - including back to the Earth's surface.
Through this process, CO2 and other greenhouse gases keep the Earth’s surface 33°Celsius (59.4°F) warmer than it would be without them. We have added 42% more CO2, and temperatures have gone up. There should be some evidence that links CO2 to the temperature rise.
So far, the average global temperature has gone up by more than 1 degrees C (1.9°F):
"According to an ongoing temperature analysis led by scientists at NASA’s Goddard Institute for Space Studies (GISS), the average global temperature on Earth has increased by at least 1.1° Celsius (1.9° Fahrenheit) since 1880. The majority of the warming has occurred since 1975, at a rate of roughly 0.15 to 0.20°C per decade."
The temperatures are going up, just like the theory predicted. But where’s the connection with CO2, or other greenhouse gases like methane, ozone or nitrous oxide?
The connection can be found in the spectrum of greenhouse radiation. Using high-resolution FTIR spectroscopy, we can measure the exact wavelengths of long-wave (infrared) radiation reaching the ground.
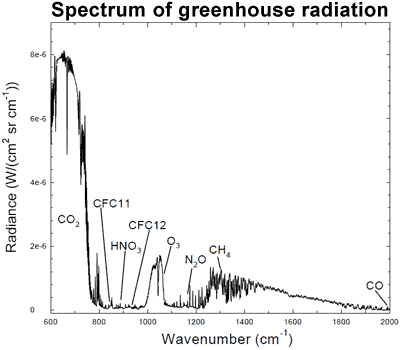
Figure 1: Spectrum of the greenhouse radiation measured at the surface. Greenhouse effect from water vapour is filtered out, showing the contributions of other greenhouse gases (Evans 2006).
Sure enough, we can see that CO2 is adding considerable warming, along with ozone (O3) and methane (CH4). This is called surface radiative forcing, and the measurements are part of the empirical evidence that CO2 is causing the warming.
...Must Go Up
How long has CO2 been contributing to increased warming? According to NASA, “Two-thirds of the warming has occurred since 1975”. Is there a reliable way to identify CO2’s influence on temperatures over that period?
There is: we can measure the wavelengths of long-wave radiation leaving the Earth (upward radiation). Satellites have recorded the Earth's outgoing radiation. We can examine the spectrum of upward long-wave radiation in 1970 and 1997 to see if there are changes.
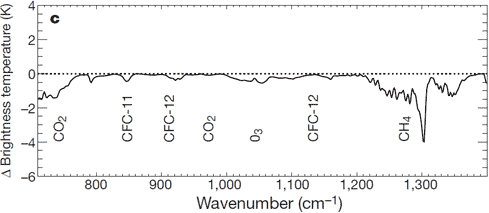
Figure 2: Change in spectrum from 1970 to 1996 due to trace gases. 'Brightness temperature' indicates equivalent blackbody temperature (Harries et al. 2001).
This time, we see that during the period when temperatures increased the most, emissions of upward radiation have decreased through radiative trapping at exactly the same wavenumbers as they increased for downward radiation. The same greenhouse gases are identified: CO2, methane, ozone and so on.
The Empirical Evidence
As temperatures started to rise, scientists became more and more interested in the cause. Many theories were proposed. All save one have fallen by the wayside, discarded for lack of evidence. One theory alone has stood the test of time, strengthened by experiments.
We have known CO2 absorbs and re-emits longwave radiation, since the days of Foote, Tyndall and Arrhenius in the 19th Century. The theory of greenhouse gases predicts that if we increase the proportion of greenhouse gases, more warming will occur.
Scientists have measured the influence of CO2 on both incoming solar energy and outgoing long-wave radiation. Less longwave radiation is escaping to space at the specific wavelengths of greenhouse gases. Increased longwave radiation is measured at the surface of the Earth at the same wavelengths.
Last updated on 16 July 2023 by John Mason. View Archives































 Arguments
Arguments






































You forgot foraminiferal ooze to go along with the carbon sink sequestration, in your example of silicate rock weathering. Venus has the advantage of the inverse square law; however, it also reflects more of the solar radiation incident upon it. It also has a sulfur dioxide concentration of 150 ppmv. Using the value I got for Carbon dioxide deg./ppmv on earth, based upon .28 deg/404 ppm and ignoring the other differences we would expect that CO2 alone on venus would contribute to a surface temperature of 669 deg C above its blackbody temperature of 226.6 K; but it is only 510C. So clearly, my estimation is not too high, if this is the route you want to take. I am within 76% agreement. what is the agreement that the climate scientists have predicted the earth's temperature to rise for the corresponding rise in CO2?
[PS] This is not "comparing with observations": it is dangerously close to sloganeering. What you have to do is you use your physical model and from it derive the say the surface temperature of venus; or the lapse rate on earth; or better still what the observable spectral signature of DLR or OLR would be under your model, and compare it what it observed. Do it for observations with different water vapour. Agreed that this is not suitable for a blog comment so do the math, put it up somewhere and post a link to it here. (And as for forminiferal ooze please learn some basic chemistry though this is a common mistake)
275 ad hominem Rob. There is no benifit to have this in a scientific discussion; it comes across as an attempt at forcing a model that has failed on its predictions to fit in the true/true square of the truth table, when all along it was the false/true square. In the scientific truth table a true hypothesis will always give rise to a true prediction; whereas a false hypothesis may give rise to a true or false prediction. It could also be that the evidence coming from the experiment may either be true or false. In otherwords, you can get evidence that will seem to support your hypothesis, even though your hypothesis is wrong. I think it is well established that we all have the same hypothesis; carbon dioxide traps in IR photons and sets a new equilibrium for the rate of incoming solar radiation and emitted blackbody radiation from the surface. The disagreement is in the value for this equilbirium. For the past half century, Scientists have performed simple enough experiments that measure the differences in radiance of peak IR absorption for CO2 at the surface and at TOA. I think they forgot to include an effect similiar to compton scattering, only not with x-rays, rather with IR waves. Water molecules in the liquid state can absorb these rays. The liquid surface can absorb rays reflected to it, and liquid in condensation nuclei of clouds can absorb rays passed through them. Ignoring this feature can lead to the appearance that CO2 is trapping in more heat than it actually does. Of course time holds the answer, securely locked away behind the wizzards curtain, in a time capsual box. The box gets opened when predictions come true. We have not melted the Arctic, we have not risen the seas, we have not caused California to stay in a drought, we have not been able to maintain an ever increasing pattern in the temperature anomaly. There have been pauses and there is going to be a huge one this year. It has already started. So do observations support my results. YES! They even work well with Venus.
[JH] Excessive repitition and sloganeering snipped.
Please note that posting comments here at SkS is a privilege, not a right. This privilege can be rescinded if the posting individual treats adherence to the Comments Policy as optional, rather than the mandatory condition of participating in this online forum.
Please take the time to review the policy and ensure future comments are in full compliance with it. Thanks for your understanding and compliance in this matter.
I did derive a surface temperature of Venus from the coefficient of heating for carbon dioxide that I determined using earth's values. I got (.0007 deg/ppmvCO2 x 965000 ppmv) CO2 and got 676 deg above blackbody temp. If you look up the black body temp of Venus it is -46.4 deg. C. So the surface temp would be 630 degrees C. According to Nasa it is 464 deg. C. I am unaware of sloganeering.
The lapse rate on earth is not a function of carbon dioxide.
Rudmop @278 now sets an acceptable standard for confirming his theory as being a 22.5% error in predicting the surface temperature of Venus. In the meantime, he considers a less than 0.5% error in predicting the absolute global mean surface temperature of the Earth as an example of model failure:
Rudmop @279, the lapse rate above the troposphere on Earth is almost entirely a function of radiative energy transfer, and hence of which gases absorb solar radiation at what altitude, and which gases absorb and emit IR radiation at what altitude. It is something successfull predicted by the theory you reject as far back as 1967. We await your equivalent prediction with bated breath.
Of even more interest to me is when you use your theory to partition energy absorption by wavelength and predict the observed outgoing IR radiation spectrum thereby. This was a test successfully past by the theory you reject in 1969. This particular test should be very easy for you to impliment if there is any validity in your method. Your failure to use your method to predict this observable (and observed) value in favour of predicting an unobservable value is very damning of your theory.
Rudmop @various.
May I offer firstly a question, secondly a prediction, thirdly some advice and fourthy why none of this is actually relevant to reality as we know it.
Firstly, a question at the most basic level. What is it you want here? It is not at all clear what that is. You have a grand theory. You tell us @249 that your grand theory has been sent off, submitted for publication 'Feb 21, 2016' which is before your first comment here. So why do you then add @249 “I am also ready for talks”? Your theory has been sent off for publication. Surely that is end of story.
(Of course, there has been since submission the small but significant amendment to your theory resulting from input from Tom Curtis replying to your initial comment here @SkS on a different thread. You will of course be submitting a corrected paper to the publishers, complete with proper acknowledgement for the correction.)
But if your theory has been sent off, why would you be “told by a scientist at Oak Ridge Laboratories to find answers to (your) questions on the effects of CO2 on heating the climate, to come to this site (ie SkS).”? What specific questions are you asking?
I look back at your initial comments here @SkS and I see no questions whatever! So what actually is it you want here?
Secondly, the chances are that your grand work will not be entered into the publications submission process but will be rejected at the first hurdle. But let us imagine that it is seriously considered for publication and is successful. Let us imagine it is published. What then?
There are scientists who regularly publised in the scientific press, scientists who are also misguided fools and just like you write up nonsense on subjects outside their competence. Being published scientists they do on occasion get published. It is not so difficult, especially if you chose your publication. As an example of such obvious nonsense consider Hermann Harde who is presently making a total twit of himself with his latest pack of twaddle - H. Harde (2017) 'Scrutinizing the carbon cycle and CO2 residence time in the atmosphere', Global and Planetary Change.
This is not the first time Harde has published denialist rubbish. In 2014 he published something not dissimilar in its implications to your grand theory. This was Harde's grand version of the GH effect & CO2's imact on climate - H. Harde (2014) 'Advanced Two-Layer Climate Model for the Assessment of Global Warming by CO2', Open Journal of Atmospheric & Climate Change. And what happened? If you visit Google Scholar you will find the impact of Harde (2014) has been sweet fanny adams.-
It has been cited by just five fellow-denialists in two years. Within proper science, Harde's nonsense does not even merit a serious rebutal.
Now you may feel it would be incredibly wrong when, if your grand work did somehow get published, it were to be simply ignored. But it will be because you have so far failed to do a very essential piece of work. You have to show not just that your sums add up, not just that your sums are valid (which remains work-in-progress for you): you have to additionally set out the argument as to why the sums being used by everybody else I the whole wide world are flat wrong. If you cannot do that, you are on a hiding to nothing. Your grand theory will simply be ignored.
And don't be surprised. Why should busy scientists have to spend time rebutting your nonsense. You have to convert your nonsense into compelling science. And the best of luck with that!!
Thirdly, ad hominem is something you will have to rise above if you work in science. Do not ignore people because they call you a fool. Ignore them only if they have nothing sensible to say. You say you are a scientist so you should already know this. So why then all this pathetic bleating about ad hominem? (I ask in this manner as you evidently need a lot more practice in dealing with the sticks and stones of the scientific process.)
And finally, why none of this matters a jot. Why isn't your grand theory worth a bean? It is because your grand theory rests entirely on the proposition that the GH-effect is additive. It is not additive. Do you not see all those non-linear equations you use? And on top of that there are a whole bunch of non-linear equations that you fail to use. You cannot just add them up and divide by the total to gain a CO2 contribution to the GH-effect.
Certainly one area where your model departs into pure fantasy is the effect of CO2 at altitudes where H2O is largely absent. @238 your explanation is silly and non-quantative in nature. (Indeed as I set out @242, I conld not make head-nor-tail of what you were trying to describe with your “CO2 is more concentrated at higher altitudes” description.)
In this regard, you have already dodged one piece of reality which was presented tp you @243. It is not the only fatal problem with your grand theory but I would suggest it is simpler to define than most. (Tom Curtis @281 calls this problem "very damning to your theory.") Here is the reality presented again.
So, can you provide said explanation?
[JH] Rudmop has unilaterally recused himself from posting on this website.
Please address the following molecular make up of CO2.
Easy Analogy: Everyone knows the higher you go on a mountain, the harder it is to breathe oxygen. The CO2 molecule is 30% heavier than O2 (oxygen molecules), meaning it stays closer to the earth’s surface. Ever wonder why a tree line pretty much stays the same? It is because there is not enough CO2 to sustain that type of plant life that high.
http://butane.chem.uiuc.edu/pshapley/GenChem1/L9/web-L9.pdf
Fact: CO2 absorbs in earth, rocks, water, and guess what, ice. As the earth heats up, water evaporates, ice melts, the CO2 trapped in all of these things, is released. It is an effect, not the cause. It is so easily absorbed in water, when it rains; it literally flushes excess CO2 out of the atmosphere, and traps it in soil, lakes, ice, etc.
Ever hear of a lake “rolling over”, and releasing the CO2 trapped in it? The CO2 kills everything within miles close to the ground. It does not dissipate into the upper atmosphere.
Here is an easy experiment, which can done at home. Open two cans of soda, put one on the counter at room temperature, and put the other in the refrigerator. After a couple of hours, pour each one into a separate glass. It is obvious from the carbonation remaining in the cans that CO2 releases much faster from the warmer can.
tmketner @283:
1) CO2 is an upper atmosphere gas as shown by these measured CO2 values (solid lines) at altitudes from 25 to 120 Kilometers above sea level:
(Source)
CO2 is heavier than air, and a release of cold CO2 gas will stay on or near the ground provided wind velocities are near zero. Even slight winds will cause the CO2 to mix thoroughly with other atmospheric gases up to an altitude at which collisions between molecules start becoming rare.
Just out of interest, here are the text book profiles of a variety of atmospheric gases:
(Source)
2) The ratio is now 1 in every 2,500 molecules. Regardless of the specific ratio, so what? The world is full of substances which have very significant effects with very small quantities. Consequently you cannot quote a small quantity in absence of all other data and make any conclusion about effectiveness. (If you want to discuss this point further, please do so at the linked page.)
3) Absent anthropogenic influences, the CO2 concentration is a function of the rate of volcanic release modulated by the rate of chemical weathering. This has varied over time, and in times of high CO2 we have had high temperatures. In the short term, the base concentration is further modulated by temperature, as you say. However, the rate of change of CO2 concentration relative to temperature in the gacial cycle would predict, at most a 40 ppmv increase in CO2 from the temperature increase over the last century. Using recent paleological data, the rate of increase durring the MWP, and decrease for the LIA would predict even less than that. You cannot argue from the glacial data that there is a connection and simply ignore the magnitude of the effect, but once you allow for the magnitude of the effect, it becomes very clear that the 20th century temperature increase is not the cause of the 20th century CO2 concentration increase.
For further discussion on that point, I suggest you read and then make further comments at this post.)
Some brief points:
You use the example of large scale CO2 release from volcanic lakes, and then say, "It does not dissipate into the upper atmosphere". Really? So according to you those pools of CO2 are still there? In fact these events almost invariably happen at night when the air is cold, and still enough to not dissipate the CO2, but within a few hours of dawn the CO2 is completely dissipated.
You argue that CO2 is washed out of the atmosphere by rain, which fails to explain why there is CO2 in the atmosphere at a slowly increasing amount despite all the rain we have. Indeed, you then give examples of CO2 being released from water (the cans of soda) contradicting that claim. In fact, CO2 in water will seek the same partial pressure as is in the atmosphere. That has resulted in about 50% of anthropogenic CO2 being absorbed by the ocean, but that leaves the other 50% in the atmosphere.
tmketner
"Ever wonder why a tree line pretty much stays the same?"
Because average temperature decreases with altitude, by on average -6.5 C/km. So the tree line matches the isotherm at the limit of th trees adaptation to cold.
Here is an easy experiment, which can done at home although it will take a bit more work. Get a sealable container that you can fill with a large amount of CO2. Open two cans of soda, put one on the counter at room temperature, and put the other one next to it in the container which you seal and fill with a concentration of CO2 greater than what was in the can of soda originally. After a couple of hours, pour each one into a separate glass. It is obvious from the much higher carbonation, actually higher than it was originally, from the can left in your CO2 container, that CO2 is absorbed into the liquid much faster when the air contains higher amounts of CO2.
tmketner
"Ever hear of a lake “rolling over”, and releasing the CO2 trapped in it? The CO2 kills everything within miles close to the ground. It does not dissipate into the upper atmosphere."
Then rescuers arrive later and they don't die. Hmmm, maybe it takes a short time for a concentrated amount to mix and that short period is all it takes to kill people before it mixes.
[PS] To understand that gas is dominated by diffusion, not molecular weight, you might like to look Bromine(heavy) + air experiment. As measurements and diffusion theory tell you, it does indeed dissipate into upper atmosphere.
tmketner
"It is so easily absorbed in water, when it rains; it literally flushes excess CO2 out of the atmosphere, and traps it in soil, lakes, ice, etc."
Not quite. Yes it dissolves in raindrops, where it reacts with the water to form Carbonic Acid. This in turn largely dissociates into Bicarbonate ions and and hydrogen ions - lowering the pH. Some of the Bicarbonate in turn dissociates into carbonate ions and more hydrogen ions. As a result the rainwater drop is slightly acidic. Most of the carbon exists as Bicarbonate and Carbonate with only modest amounts of CO2.
Then most of it ends up back in the ocean straight away - most rain falls on the oceans. Of that falling on land, most ends up flowing into the oceans anyway, snow only halts that process temporarily.
In the oceans (and to a very, very minor extent lakes), it becomes part of the broader carbon cycle. And some of the carbon ends up being outgassed back to the atmosphere again.
tmketner @283, you have forgotten that to be a properly controlled experiment, your second can of soda would have to be put into a refrigerator as large as the "room temperature" room, in order to properly minimise the back-pressure from the CO2 released from the liquid soda drink. Did you do the experiment that way?
Besides, the death of humans and animals near a lake which has "rolled-over" and released large quantities of CO2 ..... is something relatively unimportant. Professor Lindzen and other deniers have often reminded us that CO2 is very beneficial to the world, as plant food.
Thanks for the responses. I really want to understand this, so I am coming into this discussion with an open mind. I hope you are patient.
I did not mean that CO2 does not exist in the upper atmosphere, because of course it is a gas, and temperature, wind, volcanic activity, etc. will effect levels of concentration based on your graph. It is also trapped in water, so will exist in water vapor, clouds etc. My point is, based on my “lake rolling over” and “tree-line” statements, it is concentrated closer to the surface.
The levels of CO2 needed to cause the types of changes specified, would have to be much higher. Absorption, molecular structure, and the carbon cycle itself prevents the levels getting high enough to cause a significant change. If you throw in hundreds of volcanic eruptions where the Carbon cycle cannot keep up then maybe, but eventually it will even out. Isn’t it more feasible that the solar minimum/maximum cycles with the maunder minimums that happen control the temperature, which then affect the CO2 levels based on the things already mentioned?
Of course, over time, it will dissipate, but the length of time it takes to dissipate through re-absorption, or the concentration level enough to kill living things goes down, proves by design CO2 wants to remain in the lower atmosphere. Any of the other primary gases released in the same quantity would not have the same lethal effect. It kills everything for miles, not feet. There have been instances where houses 2 miles away, had a child survive in the top bunk, and the child in the bottom bunk died.
In the oceans (and to a very, very minor extent lakes), it becomes part of the broader carbon cycle. And some of the carbon ends up being outgassed back to the atmosphere again.”
I would think this would be correct if it falls directly into a river or the ocean, but the ease of absorption by earth, plants, rocks, ice, etc., makes that hard to swallow. Colder temperatures would slow this process greatly, where warmer temps would speed it up, going back to my original statement CO2 levels are controlled by temp, not the other way around.
Because CO2 is easily absorbed by pretty much everything, wouldn’t it be an exacerbating effect? If ice melts, water evaporates, rocks and soil dry out from the rising temperatures, wouldn’t all of the CO2 that is trapped in these things raise the levels of CO2 concentration found in the cores? The more temperatures keep rising, the more CO2 releases into the atmosphere.
I have done the experiment in my garage on cold nights and my car on cold nights. You may have a point about pressure, because I seem to get the best results when it is in my car, and no one opened the door. With my garage and refrigerator opening and closing, pressure does not come into play.
Rudmop - I'm afraid your assertions regarding Arrhenius are incorrect, as his 1896 paper clearly include a climate sensitivity estimate including feedbacks. That comes out to roughly 6C/doubling of CO2, considerably higher than modern estimates, although in his 1906 "Worlds in the Making" (quoted here) he revised that downwards to about 4C/doubling, due to errors regarding mutual displacement and concentration of CO2 and water vapor in the reference samples he received from Langley. That's well within the range currently considered possible by the IPCC, which is quite impressive considering that the stratosphere hadn't even been detected when he wrote it.
It's pretty clear that you are unfamiliar with the references you have been criticizing. And as I stated before, your arguments and predictions are contradicted by the experimental evidence.
"My point is, based on my “lake rolling over” and “tree-line” statements, it is concentrated closer to the surface."
The constant mixing ratio to 90km in the atmopshere, provided by Tom, is measured. Your contrary evidence from periodic overturn of meromictic lakes and tree lines is purely circumstantial. You hypothesize a pattern in CO2 from those observations, but the measurements disagree with your predictions, so your hypotheses are wrong. The effect of CO2 emitted by lakes and the location of treelines are easily explained by other hypotheses (lag in mixing, and temperature).
"The levels of CO2 needed to cause the types of changes specified, would have to be much higher. Absorption, molecular structure, and the carbon cycle itself prevents the levels getting high enough to cause a significant change."
This is assertion with no evidence. Measurements show O2 and N2 have little effect on IR radiation, while CO2 and H2O do. Models based on physics of CO2 predict a clear effect. You have to understand why they do so, before you can question those assumptions. Warning: those models are based on an enormous body of observations.
"If you throw in hundreds of volcanic eruptions where the Carbon cycle cannot keep up then maybe, but eventually it will even out."
Again, assetion without evidence. Annual human emissions of CO2 are in fact on the order of 100x the contribution by volcanic eruptions. The carbon cycle can't keep up with that. The increase is measured. The carbon can be attributed to humans using multiple lines of evidence.
"Isn’t it more feasible that the solar minimum/maximum cycles with the maunder minimums that happen control the temperature, which then affect the CO2 levels based on the things already mentioned?"
No. This hypothesis has been addressed and found wanting. Solar inputs are flat or declining since the temperature began increasing in earnest in the 1980s, when CO2 levels began taking off and CO2 effects rose above natural variation. Patterns of warming (night/day, troposphere/ stratosphere) have the fingerprints of warming due to greenhouse gasses. No model can recreate current temperature increases and these fingerprints from natural solar or albedo variations.
At this point. I'm going to stop, because I have barely got through a few paragraphs and addressed all your questions with links to this site that clearly show you are working on false premises and drawing false conclusions. You need to start with the data and work through the resulting theory. I suggest you list your questions and read the relevant posts. Then post questions if you still have them.
tmketner
Responding to your comment to Tom.
"The levels of CO2 needed to cause the types of changes specified, would have to be much higher" Why? You are making a quantitativestatement but not supplying any numbers for the argument.
The question is this. When a typical photon of IR radiation is emitted from the surface, are there enough CO2 molecules in the air column above it to intercept it before it reaches space? If no, it escapes easily and there is no heat trapping effect. If yes then it is trapped, its energy is added to the atmosphere and it contributes to modifying the climate state.
So there are two key numbers that you need to think about (actually 3).
Note that this does not depend on the relative proportion of CO2, its percentage of the atmosphere. So you are focusing on the wrong measure.
So the starting point for thinking about this is how many CO2 molecules are there? Not their proportion.
Pick a patch of ground and picture a square meter of ground area that photons might be emitted from. Now go up 1 meter. Thats 1 cubic meter of air. CO2 is around 400 parts per million of that cubic meter. How many CO2 molecules is that?
8,500,000,000,000,000,000,000
8 1/2 thousand million million million CO2 molecules. And the same in the next cubic meter above it and the next and the next....
So as a starting point, there are huge numbers of CO2 molecules, even though they are only a small percentage of the atmosphere.
That cubic meter contains around 20,760,000,000,000,000,000,000,000 molecules of all types.
This is the problem with trying to think in terms of ratios, proportions, percentages etc. Small percentages might seem insignificant but actually it is the absolute magnitude that matters, not the percentages.
tmketner

Next, refering back to one of Tom's earlier posts to Rudmop here.
Here is another graph from the same paper.
To explain the graph. the upper curve is direct observations from a satellite, the lower calculations from theory. The upper curve is shifted up for clarity, they are actually almost identical. Theory matching observations really, really well.
They are measuring the intensity of infrared radiation coming up from the Earth, wavelength by wavelength. The observations were taken by the Nimbus 3 satellite in April 1969
The area under the curve is how much energy is being radiated to space. And as you can see the curve isn't smooth, as we might expect. There arest chunks missing from it. The biggest one, centered at a wavelength of 15 microns, is due to CO2. Predicted by theory and directly observed before Armstrong and Aldrin landed on the moon.
So there, directly observed, is the major role of CO2, that gas that makes up only a small part of the atmosphere.
tmketner @289, the responses by Stephen Baines and Glenn Tamblynn are excellent, so that I have little to add beyond detail.
First, as is shown by CO2 measurements at altitude, the "tree-line" evidence is not related evidence at all. Tree-lines are governed by temperatures, not CO2 concentrations.
Second, here are the NH seasonal CO2 concentrations by altitude from Bolin and Bischof (1970):
You will notice the clear intercept points in late May and at the end of September. In the months between those periods, CO2 at moderate altitudes is more concentrated than CO2 near the surface due to the difference in amplitude of the seasonal cycle. (For measured data, see Figure 1, but the same pattern exists)
Here is equivalent data from Foucher et al (2011), but for a higher range of altitudes:
Again, due to the seasonal cycle, at certain times of the year, CO2 concentration is greater at higher altitudes. That is particularly the case given that the 16-18 km altitude band has a seasonal cycle with opposite phase. That is probably because, being above the tropopause, CO2 mixing occurs slowly by diffusion rather than rapidly due to convection. As a result the seasonal cycle is lagged relative to the surface cycle.
The lagged cycle, and diminishing seasonal cycle with altitude in the troposphere, shows that surface emissions and absorptions of CO2, together with rates of mixing dominate the CO2 altitude profile. Relative mass compared to other atmospheric molecules is largely unimportant.
Finally, particular in the NH, and particularly near cities, at very low altitudes there is a high concentration of CO2, but that is because these are locations where CO2 is generated. The opposite would be found over the Antarctic Ocean, where CO2 is absorbed by the ocean surface.
I point you to the evidence provided by Glenn Tamblynn. More specifically, from the evidence in Schmidt et al (2010), the effect of CO2 on outgoing radiation, as shown by Glenn Tamblyn @293, averages at 31 W/m^2, or 12.1% of the effect of incoming solar radiation. The further effect of doubling CO2 would be and additional 3.7 W/m^2 (before feedbacks), or equivalent to a 1.5% change in incoming solar radiation. That is much more than the observed changes in incoming solar radiation, and from reconstructions, is much more than the difference in solar radiation between the Maunder Minimum and the Grand Solar Maximum of the 20th century.
Yes, the higher the temperature, the higher the equilibrium point in pCO2. But anthropogenic CO2 emissions have raised pCO2 far above the pCO2 increase that would be expected from the temperature increase. As I previously noted, that increase would not be above 10 ppmv for the 20th century temperature increase, if we base our estimate on the glacial cycle. Less on other basis. Repeating the claim does not change that fact.
“This is assertion with no evidence” How can you say this? CO2 levels are at or near their lowest in Earth’s history. The Current average of 400PPM is well below the 1600PPM average for most of Earth’s history. Plants had to adapt some 30 mm years ago when it fell below 800PPM.
This is now in question due to diffuse CO2. The amount of CO2 released by the Earth alone closer to 1 billion tons per year on average, according to new research. Considering the unknown unknowns, none of the graphs above can be blamed on humans alone. There is much more study that needs to take place, before any definitive answer given. To sell this as “fact” is irresponsible.
When the Earth heats up, the CO2 released from the ocean alone is immeasurable. To blame that on humans is also irresponsible.
I just looked to the left of the comments section and noticed this website is copy written by John Cook… I am out, and will not participate in this topic any longer. It is a known fact that John Cook used less than 2% of his research/data to come up with his hypothesis on global warming. This comes from several sources who peer reviewed his findings.
My last point.
There are 3 reasons you hear CO2 causes climate change/global warming, even from very reputable scientists.
#1 (The evil reason) CO2 is one carbon molecule and 2 oxygen molecules. Everything living on this planet is made of carbon, uses/expels CO2, and/or interacts with it in some way or another. When you control CO2, you literally can control/profit from everything.
#2 (Not so evil) It is the big lie, so people change their ways. Reputable scientists know that people do not react to anything but crisis. Oil will run out someday, and if we have not changed our ways, or at least come up with alternatives, we are hosed. This is why reputable scientists support it, because you can't fund change, when people aren't spending money on it.
#3 (Affect from #1 and #2) If you are a scientist, you are way less likely to get funded from the #1 or #2 proponents, unless you are on the climate change boat. I understand #2. Read up on rivers and lakes so polluted/acidic, you will die within minutes of falling in. The problem with exhorting change based on falsehoods, puts you in the situation we are in, where facts get in the way, and the true focus gets lost.
[RH] Please read SkS commenting policy. Keep comments directed to the science. This is not the place for conspiracy theories. Accusations of manipulating peer reviewed research deleted.
tmketner @295:
stephen baines is correct. You did provide no evidence for your claim, and the evidence that has been provided (Glenn Tamblyn @293, Me @294) shows conclusively that your claim was incorrect.
The further "evidence" you no provide has no direct bearing on whether or not there is sufficient CO2 in the atmosphere to cause a significant greenhouse effect (what you were discussing).
Indeed, according to that research total geological outgassing from all sources amounts to 937 Mt/annum (second paragraph, page 343). But according to that same research, total geological ingassing is measured at 403 Mt/annum; and that probably represents a measurement error in that over time total geological outgassing equals total geologial ingassing. It follows that net geological outgassing is, at most around 500 Mt/Annum, and probably zero. In the meantime, that same research quotes a 2010 estimate of anthropogenic emmissions at 35,000 Mt/annum (page 342, 3rd paragraph). So, the best you can claim is that geological emissions are 2.9% of anthropogenic emissions; and probable net geological emissions are negligible in comparison.
A final question, why is it climate change deniers start invoking conspiracy theories as soon as it becomes clear they are on a hiding to nothing as regards the evidence?
[RH] Cognitive dissonance is a physically painful malady to sing. Probably best to refrain.
"A final question, why is it climate change deniers start invoking conspiracy theories as soon as it becomes clear they are on a hiding to nothing as regards the evidence? "
Because if your worldview is at odds with data, then that data must be wrong? Let your opinions be changed by facts is not something that naturally to any of us.
Also note that FF CO2 is differently isotopically from Volcanic CO2 as Tom has detailed before.
Tmketner,
I am curious as to why scientists working for Exxon and the other oil comapnies concluded that AGW is affecting the Earth. According to your points, they should have concluded that AGW was not caused by CO2 and would cause no harm. In addition, during the Bush aministration, when scientists warning about AGW were censored, scientists continued to warn about the dangers of AGW against their economic interest. The Trump administration rewards those who deny AGW but there are few takers.
Can you explain why scientists are so stupid that they act against their own clear economic interest and continue to claim that AGW is a problem?
I read a lot of this thread, but not all of it, an found that the disussion had moved on from the basic question of its title, to a temperature rise due to a concentration change. This is similar to something from wikipedia that says the forcing resulting from CO2 is accordiing to 5.65K*log CO2(1)/CO2(0) = change in temperature. Is this the understanding here, or has wiki got it wrong? Just trying to understand and if this is the wrong thread, I apologize in advance.
DrBill @299, the formula for the radiative forcing of CO2 is 5.35 x ln(CO2current/CO2initial). That is equivalent to 12.32 x log(CO2current/CO2initial). NOAA gives this, and formulas for the radiative forcing of other greenhouse gases here.
To determine the equilibrium response to a given radiative forcing, you need to multiply the forcing by the climate sensitivity factor. That is approximately equal to 0.8 +/- 0.4oC/(W/m2), which is what wikipedia says.