Greenhouse Effect Basics: Warm Earth, Cold Atmosphere
Posted on 29 February 2012 by Tom Curtis
Heating and Heat Flow
Some physics, everyone knows. In our daily lives we encounter the effects of physics all the time, and as a result, we know what physics predicts in those circumstances at a gut level. We may not be able to put it into numbers. We may not be able to apply it in novel situations. But we know it all the same.
One example is as simple as putting on a blanket. We know that if we want warm something up, we can increase the supply of heat - or we can reduce the escape of heat. Either is effective. If you have a pot that is simmering and you want to bring it to the boil, you can turn the heat up, or you can put on the lid. If we put on the lid, the pot will go nicely from simmering to boiling, and we don't need to turn up the heat even slightly. Indeed, if we are not careful to turn down the heat, the pot may well boil over.
Likewise, if you have two identical motors running with an identical load and speed (Revolutions Per Minute), one with the water pump working and one without, we are all physicist enough to say that the second one will run hotter. It does not matter that the energy supplied as fuel is identical in both cases. The fact that heat escapes more easilly with water circulating through the radiator will keep the first cooler. The consequence is that stopping the the water from circulating will lead second motor to disaster.
Nor do we find people who doubt this. Suppose somebody told us their water pump was broken, but that the Second Law of Thermodynamics prohibited transfer of heat from a cooler place (the water) to a hotter place (the engine block), so they'ld be fine so long as they didn't rev any faster than normal, we'ld look at them in complete disbelief. Or we would if we were too polite to burst out laughing. And if they set out cross country confident in their belief, it doesn't matter what destination they claim they're heading for. Rather, as we all know, they're really heading for a breakdown!

(Image copyright to iStock, and not to be reproduced without their permission.)
Heat Flow to Space
This physics that everyone knows is not only true of pots and radiators. It is true of the Earth as well. The Earth is warmed by our remarkably stable Sun. As a result, the Earth's surface radiates energy to space, and over time the incoming energy balances the outgoing energy. The process is made more complicated, however, by the existence of Infra Red (IR) absorbing molecules in the atmosphere.
Without those molecules, Infra Red radiation from the Earth's surface would travel directly to space, cooling the Earth quickly and efficiently. At certain wavelengths of Infra Red radiation, however, those molecules absorb many, or all, of the photons emitted from the Earth's surface. That energy is often redistributed among other molecules by collision, but eventually some of the redistributed energy will be reradiated by the Infra Red absorbing molecules. This process absorption, redistribution and then re-emission may occur many times before the energy escapes the atmosphere, but eventually it will either by being emitted to space, or back to the surface.
Intuitively, the energy that goes through multiple stages of absorption, redistribution and re-emission will not escape to space as fast that which is emitted directly to space from the surface. This intuition is sound, but it depends essentially on one factor, the temperature of the atmosphere.
We can see this by considering a fundamental law that governs the radiation of energy, the Stefan-Boltzmann Law:
![]()
In words, that is J-star equals epsilon sigma T to the fourth power, but we don't need to worry about that. What we need to notice is that J-star, which is the energy radiated over a given time from a given area, is proportional to the fourth power of T, ie, temperature. If the temperature doubles, the energy radiated increases sixteen-fold. If it triples, it increases eighty-one- fold. And so on. So, if the temperature of the atmosphere is different from that of the surface, the absorption, redistribution and re-emission of IR radiation by molecules in the atmosphere will certainly change the rate at which heat escapes to space.
Higher is Colder
There is another piece of physics everyone knows. It is that as you go higher in the atmosphere, the atmosphere gets colder. That is the reason why some mountain peaks are snow covered while their bases are still warm. This is not a universal law. It is not true, for example, in the stratosphere where the absorption of UltraViolet radiation from the Sun causes temperatures to rise with increased height. But eighty percent of the Earth's atmosphere is in the troposphere (the lowest layer of the Earth's atmosphere), and most radiation leaving the top of the troposphere escapes to space. And in the troposphere, as you get higher, the temperature gets lower. On average, the temperature drops by 6.5 degrees C for every thousand meters of altitude you climb. That means, for example, that the temperatures fall by about 24.5 degrees C as you climb to the summit of Mount Fuji, and by 50 to 100 degrees as you rise to the top of the troposphere.

We have already seen that temperature significantly effects the radiation of heat. Colder objects radiate less energy, and the Infra-Red absorbing molecules in the atmosphere are colder than the surface. Therefore it is no surprise that the Infra-Red absorbing molecules in the atmosphere radiate less energy to space than they absorb from the warmer surface. That difference is the essence of the greenhouse effect.
No More Arm Waving
It would be helpfull to recapitulate at this point. So far we have noted four simple facts:
- That if you reduce the escape of heat, but do not reduce the incoming heat, things warm up;
- That the atmosphere contains molecules that absorb Infra-Red radiation;
- That radiated energy depends on the temperature of the radiating object; and
- That the atmosphere gets cooler as you get higher, so that the Infra-Red absorbing molecules in the atmosphere radiate less energy to space than they absorb from the surface.
These four facts imply the existence of an atmospheric greenhouse effect, ie, that the presence of Infra-Red absorbing molecules in the atmosphere results in the surface being warmer than it otherwise would be.
In science, however, purely verbal reasoning like this is considered suspect. The reason is that sometimes odd effects occur that render verbal reasoning moot. So in science, there is no substitute for putting the theory into a mathematical form. It gets rid of the arm waving.
Fortunately for us, scientists have already put this theory into mathematical form, at a very detailed level. We can access this work, free of charge, by using the Modtran Model. The Modtran Model shows the radiation up or down over a column of atmosphere under particular conditions. By changing the conditions, you can explore the predicted effects of those changes on upward or downward radiation at any level of the atmosphere from 0 to 70 kilometers altitude. Setting the altitude to 70 kilometers effectively shows the radiation upward to space from the top of the atmosphere, or downward from space at the top of the atmosphere. Setting the altituded to 0 kilometers effectively shows the radiation upward, or downward at the surface.
Using Modtran, I determined the energy output looking downwards from an altitutude of 70 kilometers using the US Standard Atmosphere (1). The result can be seen on the following graph as the green shaded area. I repeated the model run, but this time with the altitude set at 0 km. The result is shown by the outer curve defining the red area in the graph below. That means that the red area itself, which is the upwards radiation from the surface minus the upward radiation to space, is the reduction in energy radiated to space because of the presence of Infra-Red absorbing molecules in the atmosphere. That is, it is the greenhouse effect.
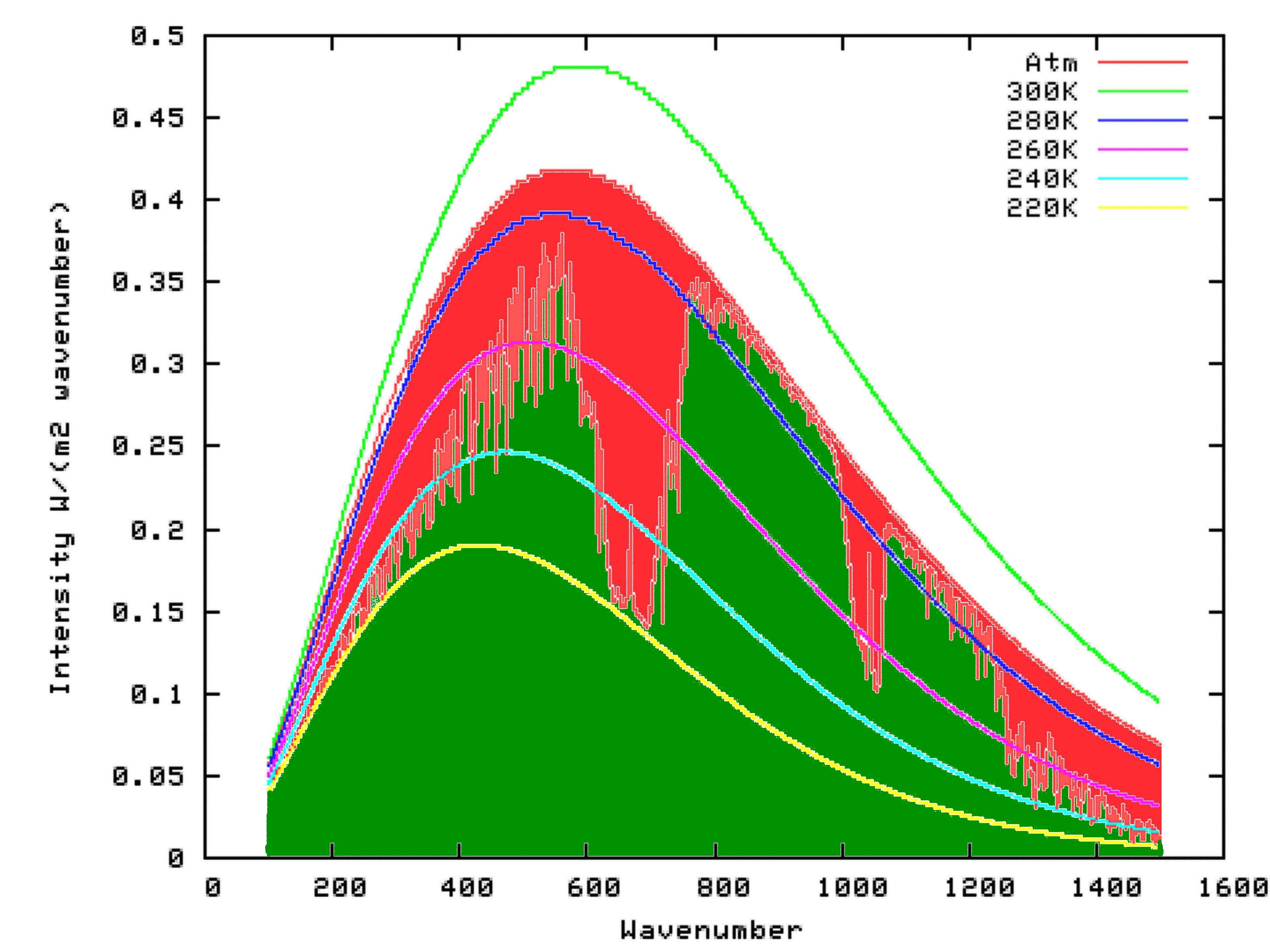
Settled Science
We have all heard how inaccurate models can be. Therefore the fact that a particular model predicts this difference in radiation only shows what the theory predicts. It does not show what is actually happening.
Scientists are not happy with theories whose only support is a model. So in 1969, Conrath and associates compared the results of model calculations of radiation to space with the actually observed radiation using the IRIS instrument on the Nimbus 3 Satellite. The following graph shows the result of their comparison. The dotted line shows the modelled values, while the solid line shows the observed values:
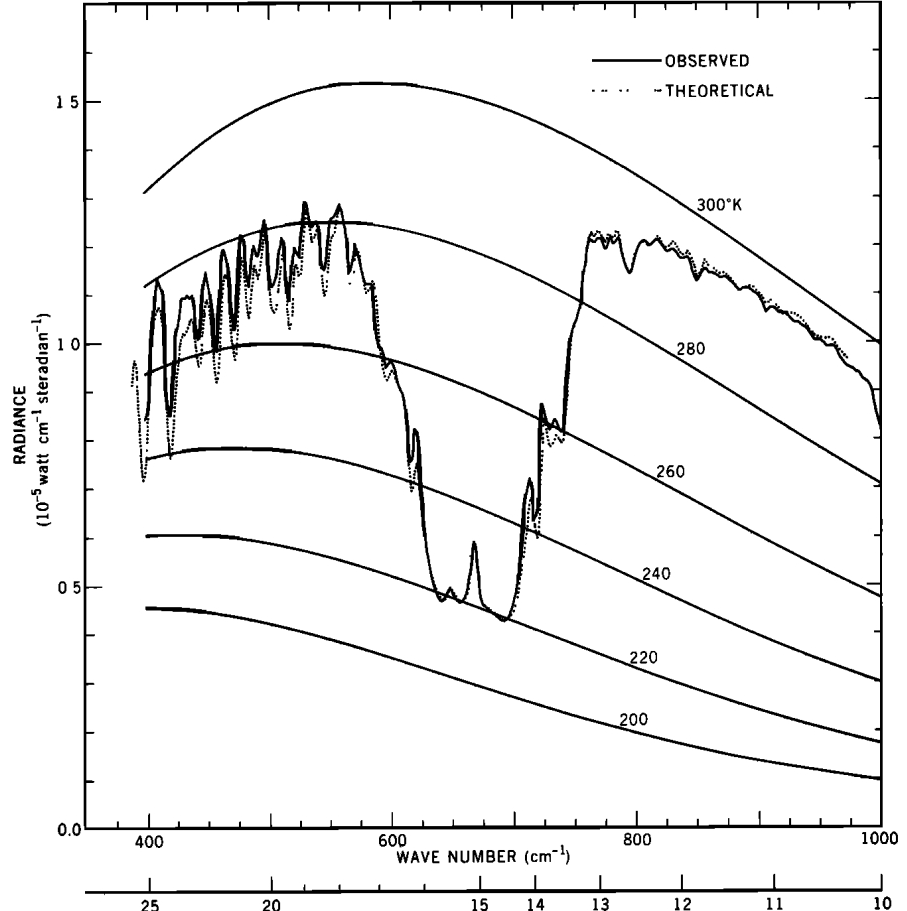
The effect of a particular Infra-Red absorbing molecule, Carbon Dioxide, is clearly visible. With the publication of this data in 1970, the greenhouse effect ceased to be theoretical. It was an observed fact.
Footnote:
(1) Default settings except for adjusting surface temperatures (Ground T offset, c) to approximately match the Earths Global Mean Surface Temperature (about -10 degrees C offset).































 Arguments
Arguments






























I have been directed to this thread but could not even start to question it, there are too many lacunae in the reasoning starting from the car engine analogy. The engine boils even if the pump keeps pumping because the heat is transported by the pump and has to be dissipated by conduction/convection through the vanes of the 'radiator' - where radiation is irrelevant, just stop the fan to find out. Radiator is a misnomer for a domestic device for conduction and convection of heat - physicists should know that.
I cannot get my head round 3 kgs of CO2 molecules accepting and re-emitting 300 watts of radiant energy in the presence of three thousand more numerous molecules of N2 O2 H2O etc - do they all agree not to collide with them so as not to convert the energy to kinetic?
I cannot get my head round the 2.9 w/m2 said to be the surplus greenhouse heating - that equates to raising the atmosheric weight of air through10 degrees p.a. - I think we would have noticed it somehow.
It's time someone addressed their energies to the way spectrographs are calibrated.
old sage - Yes, GHGs share energy back and forth with the surrounding atmosphere.
The electron relaxation time for a CO2 molecule is on the order of 10-6 seconds before radiating a photon, while at sea level pressures each gas molecule will collide ~109 times per second - meaning that a CO2 molecule will average roughly 1000 collisions before it can radiate. Therefore the GHG molecules and the surrounding atmosphere are at the same temperature.
The thing is, at thermal equilibrium the absorption spectra of an object (including a volume of gas) is equal to the emission spectra - and as much energy leaves as enters. Note that this doesn't mean the same molecules radiate as absorb, just that statistically as much energy is radiated as absorbed by radiatively active molecules in that volume. And those that radiate do so becase they have the energy to do so, because they are warm enough.
Again, you are presenting Arguments from Incredulity, in contrast to facts, to measurements. Your personal inability to get your head around those facts does not invalidate them.
Old Sage @7, this initial comment is just to clear up some (frankly silly) arguments so as to not distract from the main substance.
1) The radiator analogy: A radiator of a car in motion will not boil if it has sufficient coolant and the water pump is working. It is only if the car is stationary that disconnecting the radiator fan is sufficient to cause the radiator to boil. Further, pointing out that radiators do not loose most of their heat by radiation is irrelevant. "Radiator" remains their name, and the analogy merely points out that if you maintain a constant energy input, but decrease the energy output, the system will warm. A significant number of AGW deniers deny that basic fact.
2) The CO2 concentration in the atmosphere is measured in parts per million by volume (ppmv), not parts per million by mass. The atmospheric concentration is now 400 ppmv, so it is 4 moles of CO2 for every 9996 moles of other gases; or if you like 1 molecule of CO2 for every 2499 molecules of other gases.
3a) Your quotation of 2.9 W/m^2 forcing ignores the forcing from aerosols, which is negative. Therefore it overstates the total forcing by nearly a factor of two. Further, it is the forcing, which is the difference in top of atmosphere (TOA) radiative imbalance for a given change of radiative conditions prior to any responses to that change, including increases in temperature. Feedbacks and increases in temperature will further alter the TOA energy imbalance, with positive feedbacks increasing it, and negative feedbacks and increases in temperature reducing it. As it happens, the increase in temperature since 1750 (the reference date for forcings) has reduced the TOA energy imbalance to 0.6 W/m^2.
3b) The energy increase caused by the greenhouse effect is distributed among all Earth's surface components. That includes the upper few meters of soil, the melting of snow and ice, the increase of temperature of the ocean and the increase in temperature of the atmosphere. It even includes any increase of storage of chemical energy resulting from the CO2 fertilization effect, although that amount is (comparitavely) too small to consider. Of these components, the atmosphere absorbs around 2% (1.4% according to Church et al, 2011) of the heat, while the ocean absorbs over 90%.
If you want to pursue this line of argument, this link leads to an appropriate thread.
old sage
see my comment here
Old Sage @71, IMO, whether or not CO2 radiates at normal atmospheric pressures and temperatures is the crux of your argument. In fact, that CO2 does radiate in the IR at normal atmospheric temperatures and pressures is resoundingly confirmed by experiment.
Line by Line and broad band radiation models model the transfer of radiation within the atmosphere. For line by line models, the atmosphere is divided up into a number of layers. For each layer radiative transfer is calculated, with the total upward radiation at the top of that layer being the total upward radiation at the top of the next lower layer, less the radiation absorbed by the layer, plus the upward emissions by that layer. For line by line models, this is calculated seperately for each wave number. For broad band models, it is calculated seperately for groups or wave numbers (ie, the bands). All such models assume that each layer emits an amount based on their emissivity times the radiation expected for that wave number (or band) by a black body of the same temperature of the layer. The emissivity, of course, equals the absorptivity.
If CO2 did not radiate at normal atmospheres and pressures, such models would be massively inaccurate. Instead, they are stunningly confirmed by observations (see the section "Settled Science" in the main article, and my comment number 42). These models have not just been confirmed by observations from space, but also by observations by aircraft looking both upwards and downwards at various altitudes. Indeed, they have also been confirmed by aircraft observations looking sidewards, as the original research was done in the interests of developing accurate Infrared guided air to air missiles. They have also been confirmed by observations looking upwards from the ground. Here (courtesy of Science of Doom) is a comparison between modelled and observed back radiation:
Science of Doom has more graphs of measurments of back radiation on this page.
The back radiation is particularly devestating to your theory. As I understand it, you claim that CO2 absorbs, but does not reradiate IR radiation, except in the "electromagnetic soup" at the top of the atmosphere, ie, the ionosphere. If that were the case, there would be no IR back radiation. Any IR back radiation from the ionosphere would be as completely absorbed by the intervening atmosphere as would IR radiation from the surface. With no intervening radiation (according to your theory), the result would be a complete lack of IR radiation at the surface at bands where CO2 was strongly aborbing. Instead, we see the opposite, with the strongest IR back radiation at those wavelengths where CO2 is most strongly aborbing:
Further, nearly all of that back radiation comes from the lowest km of the Earth's atmosphere. For that reason, typically, the brightness temperature, ie, the incoming energy normalized by black body radiation curve, closely matches the surface. The exceptions are when the upper troposphere is signicantly warmer than the surface (as with Antarctica in the winter) which results in warmer wings (where CO2 is less absorptive, and hence originates from higher in the atmosphere in the case of back radiation) then does the more strongly absorbing center:
In contrast to your theory, the theory that CO2 radiates IR at normal atmospheric temperatures and pressures results in not just accurate predictions of the total energy radiated, but accurate predictions of the detailed profile of the emission spectrum given knowledge of the atmospheric temperature profile. (For the upward case, see the section "Settled Science" in the main article.
In contrast to this mass of detailed prediction and confirmation, you offer us a counter theory which has not even reached the back of envelope calculation stage. There is a reason that it has gone no further. If you take it further it immediately breaks down and is shown to be contradicted by the evidence. Given that, your choice at the moment is very clear. Embrace science by rejecting the nonsense you are currently espousing - or show clearly that it is pseudo-science you love and expouse by repeating the same old nonsense yet again.
@72
KR, Please help me understand which excited electronic states are playing a role in the greenhouse effect. I only familar with the IR vibrational quantum states.
Much appreciated.
MThompson - Those IR active vibrational quantum states are exactly what is involved in the greenhouse effect. [ For those not familar, nothing like the classics as a starter: Martin and Barker 1932, The Infrared Absorption Spectrum of Carbon Dioxide, is a good place to look ]
Those IR active vibrational states (which exclude lengthwise compression/expansion vibrations, as they don't change the electronic moment of the molecule and hence don't absorb/radiate) absorb/emit thermal range EM, with multiple wavelengths in each from different excitation states. These are further expanded by various spectral broadening effects (too many to briefly list).
Beyond that, I'm not certain what you are asking. Any IR active gas can and will act as a greenhouse gas, restricting radiation to space to an altitude where the remaining gases above have something less than a 50% chance (to a first approximation) of absorbing a particular upward photon - and due to the lapse rate, that altitude will be cooler than the surface atmosphere, meaning less energy radiated to space than would be the case in an atmosphere transparent to that wavelength. The overall effect is just a reduction in effective emissivity of the surface to space, and hence a higher temperature required to radiate the incoming energy back out, to maintain conservation of energy.
KR and MThompson,
The excited electronic states of CO2 do not play a particular role in the Greenhouse effect. Any molecules that are in excited electronic states will have slightly different vibrational frequencies and so will add to the breadth of a vibrational band. However the fraction of molecules in excited electonic states will be small (as given by the Boltzmann distribution)
I wonder whether MThompson was refering to KR's comment about "electron relaxation time" @72 which I must admit does seem out of place
Phil @78,
Thank you for your answer. I was indeed inquiring about KR@72 statement that "The electron relaxation time for a CO2 molecule is on the order of 10-6 seconds ..."
Furthermore, it seems that 10e-6 seems much too slow for relaxation of electronic states, and rather too fast for IR vibrational states.
Gratitude for furthering my education on this,
-M
Mea culpa, I often work with electronic state changes and that is my default vocabulary (in error in this case). Those are more visible/UV in range.
Vibrational (near/far IR), rotational (far IR/microwave) and combinational modes are involved in thermal IR. Radiation times for these modes are on the order of 7-15*10-6 seconds at 1atm.
Many thanks to Phil and KR for educating me on this topic. Naturally I have done considerable reading online, but many times the explanations are not clear to me because of the misapplication of concepts and poor analogies.
From my reading it seems that asymmetric stretch is the primary vibrational mode for CO2 and is some 13x more intense than the two bending modes combined. So in my understanding from this discussion and other reading is that CO2 is capturing the blackbody radiation of the earth at these wavelengths. Additionally there is some broadening of the lines that allows more than just the two primary “peaks” to be absorbed, and that broadening increases the total energy stored in vibrational modes of C02.
Please let me know if I have a good mental image of the process.
MThompson @81. I think you are both right and wrong. The asymmetric stretch is stronger than the bend, however the important fact you are missing is the distribution of IR radiation emitted by planet Earth. This is a (near) black body distribution, and the peak (at 288K) almost co-incides with the CO2 bend. Thus the bend plays a more important role simply because there is more radiation in the Earths emission spectrum to absorb.
It might be helpful to visualize the various vibrational modes of CO2 with these animated GIFs:
Molecular visualizations of CO2 from the GIF's (from Timothy Chase's website):
Ground State Mode
Pure Symmetric Stretching Mode
The pure symmetric stretching mode v1 of CO2. While this is a mode that may gain and lose energy collisionally it is not infrared (IR) active as there is no transient electric dipole.
Bending Mode V2
The bending mode v2 of CO2, responsible for the 15.00 μm (wavenumber 667 cm-1) band -- the mode dominating the enhanced greenhouse effect and that primarily used by AIRS. This is infrared (IR) active due to a transient dipole: bending results in charge being asymmetrically distributed with net positive near the carbon atom and negative near the two oxygen atoms.
And
Asymmetic Stretching Mode V3
The asymmetric stretching mode v3 of CO2 is responsible for the 4.26 μm (wavenumber of 2349 cm-1) band. The asymmetic stretch result in a net positive charge near the carbon atom and a net negative charge with the isolated oxygen atom, creating an electric dipole and making it infrared (IR) active. Given the range of atmospheric temperatures and concentrations of CO2 the bending mode v2 plays a greater role in climate change.
KR @80
Can you give a reference for your stated radiative lifetimes? I thought spontaneous emission in the mid IR (at the CO2 bending mode) had lifetimes on the order of milliseconds, not microseconds.
Phil @82
Daniel @84
Thanks for pointing out how the energy distribution factors into this. After reading your comments I did quite a bit of poking around online to refine my understanding. The big CO2 peaks may overlap some with molecular water, but the CO2 components seem to span a wavelength range of roughly 13-18 microns. Does this range correspond to higher quantum number states of the bending mode? It seems transitions between asymmetric stretch and bending mode have wavelength of about 9.5 microns.
MThompson @86,
At atmospheric temperatures only a few percent of CO2 molecules are in the first vibrationally excited state. All the rest are in the vibrational ground state. Thus, vibrational transitions to higher levels are not involved. The broadness of the bending mode comes from the fact that each vibrational state has a large number of rotational levels populated, and vibrational transitions can be from a lower to a higher rotational level ("R-branch" transitions) or from a higher to a lower rotational level ("P-branch" transitions). The rotational component of the one vibrational transition can broaden the spectral absorption band by hundreds of wavenumbers. These ever more wide-spread transitions, by the way, are why the CO2 absorption band (and water bands, for that matter, never fully "saturate" with increasing levels of water and CO2 in the atmosphere.
tcflood @87
Thank you for the clear explanation of how the rotational modes broaden the primary transitions. I am still trying to understand how the bending vibrational mode of CO2 gets populated. I see from the Maxwell-Boltzman distribution that about 6% to 15% of gas molecules at earth temperatures have enough kinetic energy to excite the CO2 bending mode. Now my question is: “Do photons from the earth’s blackbody spectrum in the range of 13-18 microns ( 770 to 560 cm-1) pump ground-sate CO2 molecules to the bending mode?”
MThompson @88;
How black body-like the earth's emission is depends on the details of the specific piece of surface, but on the average I think the consensis is that it is about 90% BB-like. So, yes, the earth's IR emission in the region around 700 cm-1 is absorbed efficiently by the CO2. The key point here, though, is that in the troposphere that excited CO2* (* means excited state) undergoes about 10^10 collisions each second and the excitation energy is transferred at about that rate to all the gases in the immediate vacinity, thus, contributiong to the thermal pool. The rate of spontaneous emission in the mid IR range tends to correspond to lifetimes of the excited state on the order of milliseconds, which is way too long for the specific originally excited CO2* (lifetime of a few hundred picoseconds) to have any probability of just emitting the photon directly back out. Thus, the low equilibrium percentage of CO2* in the atmosphere can essentially be thought of as coming entirely from the Boltzmann thermal equilibrium and this is the population from which re-emission of the IR occurs.
It sounds like your population of from 6-15% of CO2* may have been calculated assuming the ground state is singly degenerate. I think the ground state and excited state are both doubly degenerate for the bending mode so the percentages may be half of that. I'm not sure on this point. Maybe someone else can comment.
tcflood,
You are Way over my head. I'm but an interested dilettante in this man-made global warming stuff. Still, I have some observations which may or may not be pertinent to anything. Consensus: Who was polled to establish the so-called Consensus? Climatologists? Weathermen? Physicists? Sociologists? Petroleum Engineers? Volcanologists? Ecologists? Paleontologists? Archaeologists? Pathologists? Dentists? For sure, nobody polled me.
Also, science isn't about consensus. Science is the effort--sometimes the painful effort--to get at something approaching the truth. At one time "consensus" had it that the four humors were responsible for health and disease. At one time "consensus" had it that the sun revolved around the earth. At one time "Consensus" had it that most cancers started with one great mutational 'hit'. "Consensus" is a misleading term if there ever was one.
So much for my soap box. The other day I was watching a TV show--the source of most of my scientific information. The Journalist was interviewing scientific types. One was a young woman digging away in the melting Alaska permafrost. They filmed impressive looking sink-holes caused by melting ice. She climbed down into a sink-hole [looked risky to me] and showed melting frozen earth, 2-4 feet deep, containing clusters of roots from "plants that died hundreds or thousands of years ago."
Hmmm. Either these were the roots of plants that could grow in solid ice OR climate was a lot warmer in the far North way back then. [Little Climatic Optimum?]. How could it have been warmer 'hundreds of thousands of years ago, when it's supposed to be warmer NOW than since, if not before, the last interglacial? What do you think?
I know. The exception proves the rule. Still, they went on to claim that, at the present rate, by the end of the century, atmospheric CO2 would be twice that of today and the fish would boil in the sea [that's a joke]. Anyway, I googled it and did some high school arithmetic. Maybe I made a couple of systematic errors but, looks like, if we burned All petroleum and natural gas tomorrow, we would increase the tonnage of CO2 in the atmosphere by .001%. Note, this isn't saying that the % in the earth's atmosphere would go up that much. It means that atmospheric CO2 would go up a tiny fraction--IF--we burned it all at one time. Of course, all that petroleum-produced CO2 might hug the ground and heat up the surface a lot because, as we know, manmade CO2 is a lot different from 'natural' CO2.
Also, I worry a lot about carbonates. I live on a hill loaded with sea shells, ammonites, snails etc. that died tens of millions of years ago. The 'turn-over' rate is pretty slow and my guess is that they'll be locked in the rock another 65 million years. It occurs to me that the same thing is happening in the sea today. Sea life--especially those with shells--must be locking up plenty, plenty of carbonates. Once locked, they are generally fixed unless cooked and vaporized by volcanoes.
[DB] In addition to the sage advice already given you below, please read The Big Picture thread for background...and familiarize yourself with this site's Comments Policy.
Spoonie,
At this site we like people to post on topic to the thread. Since you have so many points you are off topic on most threads. Pick the one ot two you feel most strongly about and ask about that.
I noticed your high school math teacher was way off base. The atmosphere is currently 400 ppm CO2 and went up 3 ppm last year. That is about a 1% per year increase at current rates of emission. About half the emitted CO2 is absorbed so about 2% per year is emitted. There are several hundred years of supply at current rates of emission. You are off by about a factor of 1,000,000. I suspect the rest of your information is about as current as your CO2 emissions. Ask questions about what you do not understand and people will try to help you. If you get your information from the denier blogs you will stay a million times off.
spoonieduck,
As Michael suggested, you should identify the appropriate thread for each of your varied comments so that they can be addressed individually in the correct place. For your questions about Consensus, for example, you could go to the top-left of the page, where the thermometer graphic lies under the heading "MOST USED Climate Myths and what the science really says...", and you'll see that number 4 is "There is no consensus". Click on that link and it will take you to a post with Basic, Intermediate, and Advanced levels that answer your questions before you even asked them.
For the question about whether it was a lot warmer in the far North "way back then", you could start with number 1, "Climate's changed before", and learn how it's precisely that which helps us predict what the consequences will be this time. (Note that being warmer "hundreds of thousands of years ago" is not inconsistent with it being warmer now than since the last interglacial.) You might also want to read the series of posts starting with The Last Interglacial - An Analogue for the Future?
tcflood @89
So if I understand correctly, the CO2 bending mode is continuously populated by the earth's blackbody radiation in the range of 13-18 microns (770 to 560 cm-1) plus, to a lesser extent, collisions with other atmospheric gasses that have sufficient kinetic energy to activate the bending mode. Of course the most probable speed of the atmospheric gasses does not have enough energy to excite the bending mode, but some small number of atmospheric gas molecules do because of the Maxwell-Boltzmann tail.
Once the CO2 bending mode is activated (by either mechanism) it will relax primarily through collisions with N2, O2 and Ar, thus raising their kinetic energy. The probability of CO2* emitting a photon in the range of 13-18 microns (770 to 560 cm-1) is very small because of the high collision frequency of atmospheric air molecules in the troposphere.
MThompson at 93;
The rate of emission of IR photons from the excited bending mode of CO2* in the atmosphere is a property of the bulk steady-state concentration of that state regardless of the lifetime of the state for any single molecule. By my calculation using the Boltzmann equation, at 80 F about 4% of the CO2 molecules are excited in the bending mode. The rate of spontaneous emission from the sample depends on that number.
tcflood at 94,
Thanks very much. My percentages were for the energy of all gas molecules that had enough energy to excite CO2, but your steady-state 4% number is more direct and to-the-point.
Now to continue developing my mental image, the photons from the earth's surface blackbody radiation in the range of 13-18 microns (770 to 560 cm-1) are pumping the CO2->CO2* transition. The CO2* relax in one of two ways: by colliding with other atmospheric gas molecules and thus raise their kinetic energy, or the CO2* relax by releasing photons in the range of 13-18 microns. Is this a good visualization?
MThompson at 95;
I don't know that this is all going to matter in understanding the greenhouse effect, but to get the clearest physical picture of what is going on, it is probably best to separate the two phenomena of (1) IR pumping of the CO2 bending mode which has the effect of heating the surrounding atmosphere and (2) the temperature-dependent equilibrium between the vibrational groundstate (v0) and the first excited state (v1) which leads to a steadystate concentration of v1 from which IR emission occurs.
In (1) photoexcitation forms a v1 CO2* in a single specific molecule which then has a lifetime of only picoseconds because it undergoes collisional energy transfer reforming v0 CO2 and distributing the energy into the local atmospheric vicinity. Photoexcitation can be thought of as only causing atmospheric warming.
In (2) the thermal pool of all the atmospheric gases has enough energy to cause, say, 4% of the total CO2 population to persist as a constant concentration of v1 excited state species in accordance with the Boltzmann equation. Now, spontaneous emission is a strictly first-order kinetic phenonenon so that the rate of emission depends on a constant times the concentration of the excited state. The rates at which individual molecular excited states are thermally produced or thermally quenched don't matter -- only the concentration matters for photon emission.
If you are able to view the system as having two independent processes in this way, it may be easier to understand.
MThompson;
It just occurred to me that a good example might be to suppose that the GHG of concern were benzene. Let's suppose that the molecular vibration of interest were the C-H stretching mode at about 3000 cm-1. Let's say the earth somehow naturally emitted significant IR energy at that frequency. The benzene C-H stretch would be excited and that energy would be immediately transferred to the surrounding O2 and N2 thermal bath. Suppose that the ambient atmospheric temperature were about 40 C. The Boltzman distribution for that vibrational mode would be ~100 % v0 and 1 x 10^-6 % of v1. With no significant concentration of v1 at equlibrium, no emission of IR at 3000 cm-1 would be seen (say, back-radiation toward earth) from this system.
tcflood from comments 96 and 97,
Thanks. The explanations that you have given are helping me a lot. Now I see that without CO2 the earth’s blackbody radiation in the range of 13-18 microns (770 to 560 cm-1) would be able to escape to space, instead of pumping the v2 CO2* state and quickly distribute the energy into the local atmospheric vicinity.
Digging into this a little more, I found a nice calculator online that shows the earth’s BB radiation in the bandwidth of interest is about 3x1022 photons per second per square meter. I estimate that near the earth’s surface there are about 1x1022 C02 molecules per cubic meter. I have not yet had time to delve into the photoabsorption cross-section of CO2, but I’d guess that a few tens of meters would be sufficient to absorb all the earth's photons in the wavelength band of interest. This guess of course assumes that the lifetime of v2 C02* is much shorter than one second.
MThompson at 98:
You are now getting into more detail than I have, but a few tens of meters is exactly the kind of distance that I have heard or seen mentioned many times.
MThomson,
you appear to inching toward this myth. If you are, you should take the conversation to that thread.