Stratospheric Cooling and Tropospheric Warming - Revised
Posted on 18 December 2010 by Bob Guercio
This is a revised version of Stratospheric Cooling and Tropospheric Warming posted on December 1, 2010.
Increased levels of carbon dioxide (CO2) in the atmosphere have resulted in the warming of the troposphere and cooling of the stratosphere which is caused by two mechanisms. One mechanism involves the conversion of translational energy of motion or translational kinetic energy (KE) into Infrared radiation (IR) and the other method involves the absorption of IR energy by CO2 in the troposphere such that it is no longer available to the stratosphere. The former dominates and will be discussed first. For simplicity, both methods will be explained by considering a model of a fictitious planet with an atmosphere consisting of CO2 and an inert gas such as nitrogen (N2) at pressures equivalent to those on earth. This atmosphere will have a troposphere and a stratosphere with the tropopause at 10 km. The initial concentration of CO2 will be 100 parts per million (ppm) and will be increased to 1000 ppm. These parameters were chosen in order to generate graphs which enable the reader to easily understand the mechanisms discussed herein. Furthermore, in keeping with the concept of simplicity, the heating of the earth and atmosphere due to solar insolation will not be discussed. A short digression into the nature of radiation and its interaction with CO2 in the gaseous state follows.
Temperature is a measure of the energy content of matter and is indicated by the translational KE of the particles. A gas of fast particles is at a higher temperature than one of slow particles. Energy also causes CO2 molecules to vibrate but although this vibration is related to the energy content of CO2, it is not related to the temperature of the gaseous mixture. Molecules undergoing this vibration are in an excited state.
IR radiation contains energy and in the absence of matter, this radiation will continue to travel indefinitely. In this situation, there is no temperature because there is no matter.
The energy content of IR radiation can be indicated by its IR spectrum which is a graph of power density as a function of frequency. Climatologists use wavenumbers instead of frequencies for convenience and a wavenumber is defined as the number of cycles per centimeter. Figure 1 is such a graph where the x axis indicates the wavenumber and the y axis indicates the power per square meter per wavenumber. The area under the curve represents the total power per square meter in the radiation.
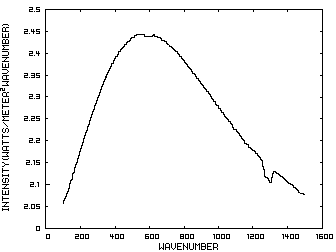
Figure 1. IR Spectrum - No Atmosphere
The interaction of IR radiation with CO2 is a two way street in that IR radiation can interact with unexcited CO2 molecules and cause them to vibrate and become excited and excited CO2 molecules can become unexcited by releasing IR radiation.
Consider now the atmosphere of our fictitious model. As depicted in Step 1 of Figure 2, N2 and CO2 molecules are in motion and the average speed of these molecules is related to the temperature of the stratosphere. Now imagine that CO2 molecules are injected into the atmosphere causing the concentration of CO2 to increase. These molecules will then collide with other molecules of either N2 or CO2 (Step 2) and some of the KE of these particles will be transferred to the CO2 resulting in excited CO2 molecules (Step 3) and a lowered stratospheric temperature. All entities, including atoms and molecules, prefer the unexcited state to the excited state. Therefore, these excited CO2 molecules will deexcite and emit IR radiation (Step 4) which, in the rarefied stratosphere, will simply be radiated out of the stratosphere. The net result is a lower stratospheric temperature. This does not happen in the troposphere because, due to higher pressures and shorter distances between particles, any emitted radiation gets absorbed by another nearby CO2 molecule.
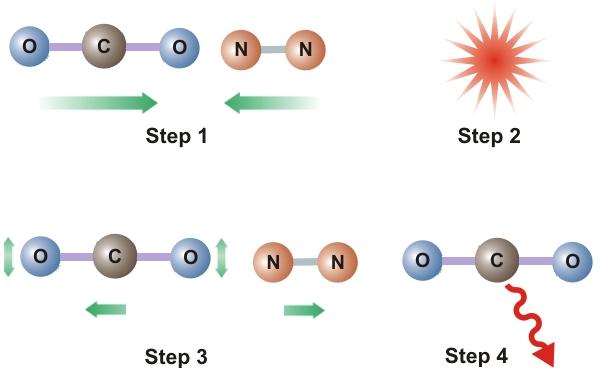
Figure 2. Kinetic To IR Energy Transfer
In order to discuss the second and less dominant mechanism, consider Figure 1 which shows the IR spectrum from a planet with no atmosphere and Figures 3 which shows the IR spectrums from the same planet with CO2 levels of 100 ppm and 1000 ppm respectively. These graphs were generated from a model simulator at the website of Dr. David Archer, a professor in the Department of the Geophysical Sciences at the University of Chicago and edited to contain only the curves of interest to this discussion. As previously stated, these parameters were chosen in order to generate graphs which enable the reader to easily understand the mechanism discussed herein.
The curves of Figures 3 approximately follow the intensity curve of Figure 1 except for the missing band of energy centered at 667 cm-1. This band is called the absorption band and is so named because it represents the IR energy that is absorbed by CO2. IR radiation of all other wavenumbers do not react with CO2 and thus the IR intensity at these wavenumbers is the same as that of Figure 1. These wavenumbers represent the atmospheric window which is so named because the IR energy radiates through the atmosphere unaffected by the CO2.
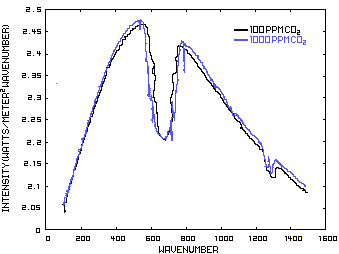
Figure 3. CO2 IR Spectrum - 100/1000 ppm
A comparison of the curves in Figure 3 shows that the absorption band at 1000 ppm is wider than that at 100 ppm because more energy has been absorbed from the IR radiation by the troposphere at a CO2 concentration of 1000 ppm than at a concentration of 100 ppm. The energy that remains in the absorption band after the IR radiation has traveled through the troposphere is the only energy that is available to interact with the CO2 of the stratosphere. At a CO2 level of 100 ppm there is more energy available for this than at a level of 1000 ppm. Therefore, the stratosphere is cooler because of the higher level of CO2 in the troposphere. Additionally, the troposphere has warmed because it has absorbed the energy that is no longer available to the stratosphere.
In concluding, this paper has explained the mechanisms which cause the troposphere to warm and the stratosphere to cool when the atmospheric level of CO2 increases. The dominant mechanism involves the conversion of the energy of motion of the particles in the atmosphere to IR radiation which escapes to space and the second method involves the absorption of IR energy by CO2 in the troposphere such that it is no longer available to the stratosphere. Both methods act to reduce the temperature of the stratosphere.
*It is recognized that a fictitious planet as described herein is a physical impossibility. The simplicity of this model serves to explain a concept that would otherwise be more difficult using a more complex and realistic model.
Robert J. Guercio - December 18, 2010































 Arguments
Arguments






























[DB] Welcome to Skeptical Science. Here we focus on the science, not on rhetoric or ideology; that focus immediately separates SkS from the other sites you reference. Please familiarize yourself with the Comments Policy of this site. Note that a continued focus on "camps" or "tribes" detracts from one's credibility here.
Inflammatory snipped.
I'm not satisfied by this story. See also how it is debunked in the comments. Let me propose a new theory here and see how people here think it adds up. The theory is somewhat similar to the story here. Maybe I am just explaining the same, but differently. My Theory:
1) About CO2:
- When a molecule collides with any other molecule, it either keeps its kinetic energy (KE) or gets in excited state (E).
- When in (E): When it collides, the excitement might get converted to KE. Or, after a while, it turns to normal state by radiating some IR.
2) About the atmosphere, for simplicity there just are:
- An upper part, Stratosphere (S), low pressure.
- A lower part, Troposphere (T), high pressure.
3) About what changes:
- The concentration of CO2 increases in both layers of the atmosphere.
- All IR still travels in all directions through both layers, but the chance of hitting CO2 is increased.
4) The explanation why S cools down and T warms up:
- In S the CO2 molecules have less frequent collisions than in T, just by the lower pressure.
- We have chance A: The chance that an excited (E) CO2 molecule radiates IR (chance A).
- We have chance B: The chance that an excited (E) CO2 molecule turns back to normal state by the next collision radiates IR (chance A).
- In S chance A is much higher than B.
- In T chance B is slightly higher than A.
Reactions are appreciated.
[DB] "See also how it is debunked in the comments"
It is not debunked in the comments.
Josbert, when you have model for how something works, then science works by making predictions from the model and comparing them to observations. Cooling of the stratosphere while warming of the troposphere falls straight out the radiative transfer equations (RTE). The RTE are widely used (think about why US Air Force are people that developed the MODTRAN codes) and their predictions about observed radiation whether observed from earth or satellite are matched in equisite detail. However, this is a "shut up and calculate" approach to science and doing an explanation without the math for non-specialists is challenging. I dont like them. However, I can assure you that you are in for an uphill battle convincing anyone that the RTEs are wrong without doing the math and showing that somehow your model produces even better match to observations.
Josbert Lonnee @128,
You present a hypothesis that relies on how a low pressure GHG gas radiates energy rather than how an increase in the GHG concentration impacts the net radiation. Here I will restrict the comment to low pressure, rather than low pressure with increased GHG.
Perhaps the hypothesis can be stood on its head and used to argue that the low pressure gas would be warmer and unable to radiate energy away as efficiently as a high pressure gas. Your argument rests on the idea that an excited CO2 molecule has more opportunity to radiate a photon as there is more time between the collisions that put it into that excited state. Conversely, once the wicked deed is done, the CO2 molecule is no longer in this excited state and thus unable to radiate a photon and that period of unexcited time is far longer at 10mbar than it is at 200mbar. Thus the frequency of (E), the A-or-B situation will be lower and the excited CO2 photons radiated by the gas would be less even if the probability A is relatively higher than B in a lower pressure gas.
Or just perhaps, these two effects cancel each other out.
@MA Rodger at 21:01 PM on 2 November, 2018
I really do not understand what you are trying to tell here, sorry. What is your point?
scaddenp @ 129
Do you understand that I am not challenging the observations that the troposphere is warming and the stratosphere is cooling?
I do understand that, but I have massive mistrust of hand-wavy models compared to precisely stated mathematical models which reproduce multiple types of observations. It isnt clear to me whether you are challenging the RTEs or trying to do a plain English explanation of the net effect.
scaddenp @ 133
Im am not doing any of both. Please, can you, based on the RTEs, tell me why my theory is wrong?
Josbert Lonnee @131,lecules
Mu point is that you are wrong. Your stated hypothesis contains a number of fundamental flaws. I set out just one (and it only requires one).
You say the probability of an excited molecule emitting a photon following a collision is increased by an increased average path-length between collisions (thus your A:B ratio increases). You suggest this increase would increase cooling in S relative to T but you ignore the lower number of collisions that the molecules endure in S relative to T, a consideration which will cancel out your A:B increase.
MA Rodger @ 135
The number of collisions between molecules stays the same, independent of the concentration of CO2. Only the CO2 molecules are less likely to pass all kinetic energy from collision to collision. So, the mode CO2 is in S, the more energy is radiated away as photons.
I still do not get this point. Do you have another? You suggest you have more points.
Josbert Lonnee @136,
Another point? Let us stick with this one. It is surely the most straightforward.
You say the number of collisions stays the same, this presumably collisions per molecule. Your thesis is that a lower pressure in the stratosphere (relative to the troposphere) provides a longer time between collisions, more time for a photon to be emitted and so there will be more photons emitted, the gas will cool more.
But if a molecule takes more time between collisions, how can there be the same number of collisions? Simply, there cannot be!!
MA Rodger @ 137
What if one would isolate a (huge) amount of air from S and make it the same temperature and put it under the same (low) pressure as there.
The theory is not that the time between collisions is (initially) longer between all O2, N2 and CO2 molecules etc. The time is just longer in S than in T because of the lower pressure. Hereby the CO2 concentration is irrelevant.
After a while, after the gas (like in S) cooled down (slightly), there will be (slightly) less molecule collisions. That makes a difference, but for this it needs to cool down first. The theory is just that the CO2 molecules took the kinetic / heat energy out by readiating it out of the isolation.
Josbert, the relaxation time for a gasseous CO2 molecule is about 10 microseconds. But during that timespan, a CO2 molecule (even in the cold lower statosphere) has roughly 10,000 collisions with neighbouring N2 (or O2) molecules.
If a CO2 molecule does "relax" to emit an IR photon, the photon can travel only a very short distance until it is absorbed by another CO2 molecule. So it is highly likely that the second CO2 molecule will lose this added energy, by collision with a neighbouring N2 (i.e. by warming the nearby air molecules).
As air density decreases, a few IR photons will be able to "miss" CO2 molecules and escape to outer space — in other words, the CO2 in the stratosphere will cool the stratosphere (while the stratosphere is being warmed by the lower atmosphere, which is being warmed from the planet surface by radiation & convection & H2O condensation).
I am unclear on how your ideas fit in with this picture.
Josbert Lonnee @138,
I am unclear about your reason for presenting your "picture."
There is no need to set up hypothetical situations. The temperature of the lower stratosphere is no different to the temperature of the top of troposphere. All that changes is the reduction of pressure with altitude.
And the CO2 levels don't vary to any significant degree through these altitudes, as these coloured traces of CO2 for altitudes 8km to 18km demonstrate.
As for photons escaping to space, that occurs mainly in the upper troposphere where the IR warming runs along side (and thus is in balance with) warming from atmospheric circulations.
Eclectic @ 139
Interesting!
Do you also mean that the stratosphere intercepts most infrared (as intercepted by the CO2 in it) and the troposphere intercepts it all?
MA Rodger @ 140
What you tell here is not new to me and the theory is based on assuming that what you say here is true.
MA Rodger @ 140
The point of the theory is to eplain why T warms up while S cools down. So I do not understand why you come up with these facts.
Josbert Lonnee @142/143,
Correct me if I am wrong. When you talk of "the theory" you are meaning that set out @128 where you describe "my theory." In it you describe a mechanism for a cooling stratosphere and a warming troposphere. You suggest this may be a re-statement of the situation described in the post at the top of this thread [although it is not]. You base your "theory" on the probability of a CO2 molecule emitting a photon following a molecular collision in which it is excited and thus able to emit a photon of IR, a probability which will be greater if this CO2 molecule has longer before it is in another collision. If more photons are emitted by a gas (per molecule), more cooling will occur.
That is what you appear to be saying.
Your suggestion is wrong in a number of ways. The most straightforward error is to consider a higher probability of a CO2 molecule emitting a photon after a collision without considering the lower probability of CO2 molecules being in an appropriate collision. Simplisitically, the two probabilities cancel out. So it is not the case that more photons are emitted from lower pressure air.
MA Rodger @ 144
It was already clear to me that you understand me all wrong all the time. With "the theory" I of course mean my theory from @128.
There is no need to repeat your point.
Josbert Lonnee @145,
While you appear to be struggling with the English language, I feel we have reached the end of this interplay. After five attempts to communicate with you regarding a blindingly simple concept, you complain that I understand you "all wrong all the time." If that were true, it is up to you to explain yourself properly. However, I do not consider it true. Your 'theory' set out @128 is nonsense and if you cannot grasp the blindingly simple idea that if CO2 takes longer to travel between collisions then there must be less collisions; if you cannot grasp that obvious truth then there is no point in my responding to you. [Mind, I will likely respond to your comments but not to you.]
Josbert @145 ,
in your [post #128] scenario, the essential point is that the rate of CO2 molecules emitting IR photons is extremely low in comparison to the rate of collisions, in both the Stratosphere and the Troposphere. In other words, the chance of a photon-emitting relaxation is (very roughly) around 0.01% compared with the chance of an intervening collision (the collision being likely to de-energize the CO2 molecule from its newly-gained photonic packet of energy).
If, owing to different temperatures and densities, that percentage should vary between (very roughly) 0.03% and 0.003% [one order of magnitude] in the two layers of atmosphere . . . then you see that the absolute difference from the [almost 100%] collision chance, remains approximately equal for "S" and "T".
Thus your #128 proposal is barking up the wrong tree. You should focus on the absolute distances ~ the mean travel distance from one "emitting-CO2" to the next "absorbing-CO2". This distance is very short, and (greatly enhanced by the relaxation "delay" period) is the reason why the appropriate IR takes a long time to rise from planetary surface up to the top of the troposphere and eventually make its escape (upwards) from there [and a smaller mount will escape upwards from the stratosphere ~ which is why increased solar irradiation of the stratosphere has a stratosphere-warming effect compared with the stratosphere-cooling effect of increased CO2 concentration [regardless of whether it's a human-caused or natural-caused CO2 increase]).
Josbert, stay on MA Rodger's good side, and he may well provide a much more rigorous explanation than I can !
Eclectic @ 147
What you say is very clear and shows my theory is false.
MA Rodger just kept mentioning the same point and that I do not understand that. My English was to blame.
Josbert, I am happy to be of help. The so-called Greenhouse Effect is rather counter-intuitive for many people (including me), and I find a deficiency of sites where it is explained completely all in one chapter. In particular, the stratospheric cooling effect is interesting, partly because it demonstrates the falsity of some of the claims of the climate science denialists [the faux-skeptics].
As you are aware, the large majority of 15-micron IR is lost from Earth at the altitude of the upper troposphere, yet a tiny amount is lost from the stratosphere itself, too.
My apologies for my typographical error ["typo"] in my third paragraph of #147, where I wrote "and a smaller mount will escape" ~ the correct word would be amount. This sort of typo must be troublesome and annoying for a reader who is not 100% fluent in English.
Eclectic @ 149
Yes, there is a deficiency of good, neutral articles on the Greenhouse Effect. I am actually trying to write something like that in Dutch. It is here:
https://josbertlonnee.wordpress.com/2018/11/20/het-broeikaseffect/
The stratospheric cooling is not mentioned there (yet). To fascinate a bigger crowd, things shoult get kept simple, but with this kind of subjects that is really hard.
I already started reading more about the IR-spectrum of the earth's atmosphere. I learned you never know enough w.r.t. climate. For instance, how exactly do molecules transfer energy from photons to kinetic energy?
I completely overread your typo. I am quite used to English; not to speaking it. Is my English really that bad?