Stratospheric Cooling and Tropospheric Warming
Posted on 1 December 2010 by Bob Guercio
This post has been revised at Stratospheric Cooling and Tropospheric Warming - RevisedIncreased levels of carbon dioxide (CO2) in the atmosphere have resulted in the warming of the troposphere and cooling of the stratosphere. This paper will explain the mechanism involved by considering a model of a fictitious planet with an atmosphere consisting of carbon dioxide and an inert gas such as nitrogen at pressures equivalent to those on earth. This atmosphere will have a troposphere and a stratosphere with the tropopause at 10 km. The initial concentration of carbon dioxide will be 100 parts per million (ppm) and will be increased instantaneously to 1000 ppm and the solar insolation will be 385.906 watts/meter2. Figure 1 is the IR spectrum from a planet with no atmosphere and Figures 2 and 3 represent the same planet with levels of CO2 at 100 ppm and 1000 ppm respectively. These graphs were generated from a model simulator at the website of Dr. David Archer, a professor in the Department of the Geophysical Sciences at the University of Chicago and edited to contain only the curves of interest to this discussion. The parameters were chosen in order to generate diagrams that enable the reader to more easily understand the mechanism discussed herein.
Prior to discussing the fictitious model, consider a planet with no atmosphere. In this situation light from the sun that is absorbed by the surface is reemitted from the surface. Figure 1 is the IR spectrum of this radiation which is known as Blackbody radiation.
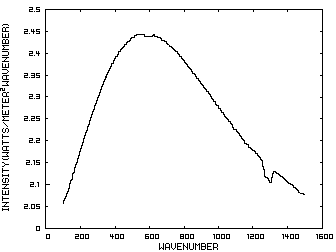
Figure 1. IR Spectrum - No Atmosphere
Consider now Figure 2 which shows the Infrared (IR) radiation spectrum looking down at the planet from an altitude of 10 km with a CO2 concentration of 100 ppm and Figure 3 which shows the IR spectrum with a CO2 concentration of 1000 ppm. Both figures represent the steady state and approximately follow the intensity curve for the blackbody of Figure 1 except for the missing band of energy centered at 667 cm-1. This band is called the absorption band and is so named because it represents the IR energy that is absorbed by CO2. IR radiation of all other wavenumbers do not react with CO2 and thus the IR intensity at these wavenumbers is the same as that of the ground. These wavenumbers represent the atmospheric window and is so named because the IR energy radiates through the atmosphere unaffected by the CO2. The absorption band and the atmospheric window is the key to stratospheric cooling.
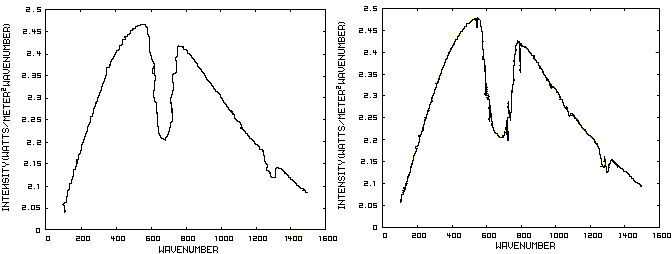
Figure 2. CO2 IR Spectrum - 100ppm Figure 3. CO2 IR Spectrum - 1000 ppm
The absorption band in Figure 3 is wider than that of Figure 2 because more energy has been absorbed from the IR radiation by the troposphere at a CO2 concentration of 1000 ppm than at a concentration of 100 ppm. The energy that remains in the absorption band after the IR radiation has traveled through the troposphere is the only energy that is available to interact with the CO2 of the stratosphere. At a CO2 level of 100 ppm there is more energy available for this purpose than at a level of 1000 ppm, thus the stratosphere is cooler for the higher level of CO2 in the troposphere. Additionally, the troposphere has warmed because it has absorbed the energy that is no longer available to the stratosphere.
One additional point should be noted. Notice that the IR radiation in the atmospheric window is slightly higher in Figure 3 than Figure 2. This is because the temperature of the troposphere has increased and in the steady state condition, the total amount of IR entering the stratosphere in both cases must be the same. That total amount of energy is the area under both of these curves. Thus, in Figure 2, there is more energy in the absorption band and less in the atmospheric window while in Figure 3, there is less energy in the absorption band and more in the atmospheric window.































 Arguments
Arguments






























tcflood,
This is a response to this comment from The Big Picture...
You asked:
Yes.
As explained earlier, there are two answers to this.
Molecular Reasons
Let's start with the first, in which there are two interlocking mechanisms at work. One is the absorption and emission of IR by CO2. The other is the transmission of energy between molecules as a result of a collision.
So in the lower troposphere, where the air is dense, there is a lot of CO2 to absorb IR. But there are also far more O2 and N2 molecules, and so many collisions, such that energy is quickly transferred through collisions to O2 and N2 molecules. What was vibrational energy in the CO2 molecule turns into translational (and perhaps very slightly rotational) energy in an O2 or N2 molecule.
Of course, everything is rate of reaction:
1) * + CO2 --> CO2*
2) CO2* + O2 --> CO2 + O2* (O2* is just accelarated, not "excited", O2)
3) CO2* --> CO2 + *
4) O2* + CO2 --> O2 + CO2*
Equation 1 happens a lot, but is dependent on the concentration of CO2 and * (IR photons in the right wavelength).
Equation 2 happens a lot more, but is dependent on the concentration of excited CO2* molecules and O2 (or N2) molecules.
Equation 4 happens pretty much, and is dependent on the concentration of unexcited CO2 and O2 (or N2) molecules.
Equation 3 happens not so much in the denser parts of the troposphere, because equation 2 happens so often and so frequently that excited CO2* doesn't have much chance to re-emit IR. Of course, it does happen, but then what matters is rates of reaction, and which equations dominate the system in which states.
As we move into the less dense upper troposphere and stratosphere, we find that there is less IR to absorb, and also with more rarefied air, less collisions. So the relative rates of equations 1 and 2 go down, while 3 and 4 go up. We reach a point where rather than absorbing IR and heating the surrounding atmosphere (1 and 2), the surrounding atmosphere is "heating" (exciting) CO2 which then is able to emit the IR (in all directions, obviously), some of which makes it into space.
So, on a molecular level, this explains why CO2 cools the stratosphere but warms the troposphere.
Emission Reasons
I've thought about this less, so my explanation will be somewhat vague... and honestly, we've recently had a discussion (argument) about both of these reasons, and which is more correct (molecular or this one, for which I don't have a good name)...
As you have recognized, the earth must emit exactly as much as it absorbs, once it achieves equilibrium. This means that at a warmer temperature it will still emit the same level of radiation, but the profile of that radiation will have changed. This can be seen in figures 2 and 3 of this original SkS post above. What it means is that the same total amount of radiation is emitted, but the spectrum will have less emissions in the "CO2 window". This in turn means that there is less IR for the CO2 in the stratosphere is to absorb (again, equation 1 happens less often in the stratosphere, but it does happen) and so equations 1 and 2 are even less likely to happen.
Summary
1. Raising CO2 in the troposphere increases the chance of IR being absorbed and transferred to the surrounding atmosphere.
2. Raising CO2 in the stratosphere increases the chance of energy being "stolen" from the stratosphere and emitted as IR into space.
3. Raising CO2 in the troposphere changes the profile of outgoing radiation, such that there is less radiation in the CO2 IR band for the stratosphere to absorb to "stay warm."
tcflood,
I just noticed that the phrasing of your question was also inaccurate. No one says the statosphere must cool for the troposphere to heat. The real fact is that the nature of CO2 (unlike other forcings) will heat the troposphere and cool the stratosphere, independent of each other, for the reasons explained above.
This is different, for example, from an increase in solar insolation, which would warm the surface and atmosphere directly, causing the earth to radiate more (more in, more out), but would leave the atmosphere unchanged (except for whatever effects the increase in incoming radiation would have directly on the stratosphere).
Or consider the case of less ice, such as when the ice sheets retreat at the end of a glacial period. Less ice reflects less radiation. With less radiation reflected in the visible spectrum, the earth will heat more, until it emits enough IR to compensate for the increased absorbed radiation. The earth is receiving and will emit the same total amount of energy, but more of it must be in the IR (and the planet is warmer). But this would have minimal effect on the stratosphere.
This is one reason why stratospheric cooling is itself a signature -- a fingerprint -- that adds one more bit of evidence that CO2 is the cause of our current warming. The theory predicts stratospheric cooling, and we see it, when other warming mechanisms would not show this.