Settled Science - Humans are Raising CO2 Levels
Posted on 20 August 2011 by dana1981, MarkR
 As a result of Murry Salby's fundamentally flawed arguments that the increase in atmospheric CO2 is a consequence of the increase in temperature, rather than vice-versa, a number of prominent climate "skeptics" have been taking up this argument (i.e. Watts, Curry, Bolt, Jo Nova). In his post on the subject, Watts wrote
As a result of Murry Salby's fundamentally flawed arguments that the increase in atmospheric CO2 is a consequence of the increase in temperature, rather than vice-versa, a number of prominent climate "skeptics" have been taking up this argument (i.e. Watts, Curry, Bolt, Jo Nova). In his post on the subject, Watts wrote
"I’m pretty sure Australian bloggers John Cook at Skeptical Science and Tim Lambert at Deltoid are having conniption fits right about now."
Indeed, we here at Skeptical Science have found the entire hubub over Salby's claims by those who claim to be serious climate skeptics rather frustrating. This is because Salby's argument is akin to claiming the Sun revolves around the Earth. We know it's wrong, we know why it's wrong, and we've known this for ages. If somebody tells you the Sun revolves around the Earth, your reaction should not be "wow, this could revolutionize the entire field of astrophysics!", your reaction should be "no, unless you produce some absolutely extraordinary evidence, that's obviously wrong." Of course, that will only be your reaction if you're a real skeptic.
Although we've addressed Salby's arguments in a few recent posts already (see here and here and here), we previously had not created a comprehensive rebuttal to the myth that the atmospheric CO2 increase is natural (though RealClimate has a good one, from which we borrowed some of the discussion on carbon isotopic signatures). Thus we have now taken the opportunity to create this rebuttal.
Simple Accounting
The easiest way to prove that the atmospheric CO2 increase is man-made is through a simple accounting approach. The equation for the change in atmospheric CO2 (ΔCatm) is
This says that if we ‘emit’ a ton of carbon by, say, triggering a volcano then the atmosphere will gain a ton. If we ‘absorb’ a ton of carbon by growing a tree, then the atmosphere loses a ton. We can expand the equation by counting human emissions (HE) and absorption (HA) and natural emissions (NE) and absorption (NA) separately.
This works because carbon is additive. If a volcano emits a ton of carbon and a factory emits a ton then the atmosphere has gained two tons. This is a very simple balance sheet for the carbon cycle and fortunately there are ‘accountants’ who have measured some of these values for us.
Recently the amount of CO2 in the atmosphere has been rising at ~2 parts per million per year, or around 15 billion tons/year. Meanwhile human emissions excluding land use change (like clearing or planting forests) are 30 billion tons per year. In billions of tons per year we have:
We can rearrange this:
Humans are also clearing rainforests and changing land use, but here we'll assume that human effects on absorption (HA) are not much different from zero, i.e.
So Natural Absorption (NA) must be bigger than Natural Emissions (NE). Nature is absorbing more CO2 than it is emitting. It is not causing atmospheric CO2 to rise at all - in fact it is acting to try and reduce atmospheric CO2, and thus the long term rise is entirely because of humans.
Ocean Acidification
The oceans are the Earth's largest carbon storage medium, so if the atmospheric CO2 increase were "natural", it would likely be coming from the oceans. But we know the CO2 increase is not coming from the oceans, because the pH of the oceans is dropping (a.k.a. ocean acidification).
When CO2 is absorbed into a solution, it binds with a water molecule to form a molecule of carbonic acid:
CO2 + H2O = H2CO3
H2CO3 has a rather strong acidifying effect in that 95% of it turns into HCO3-. This loss of an H+ ion causes the ocean pH to decrease (for more details on ocean acidification, see the OA no OK series).
In short, the fact that the pH of the oceans is decreasing tell us that they are absorbing more carbon than they are releasing, not vice-versa.
Oceanic CO2 Rising Fastest at the Surface
If CO2 were being driven into the ocean from the air, the oceanic concentration would rise fastest at the surface. If CO2 were being expelled from the oceans, we would expect to see the opposite - decreasing concentrations at the surface.
The World Ocean Circulation Experiment (WOCE) and the Joint Global Ocean Flux Study (JGOFS) has observed that as we expect for CO2 being driven into the oceans, concentrations of CO2 in the oceans are rising fastest at the surface.
Atmospheric O2 is Decreasing
Burning carbon requires oxygen (O2), and when we burn an atom of carbon, the required oxygen becomes part of the CO2 molecule. So if the CO2 increase is caused by burning carbon (fossil fuels), we would expect atmospheric O2 levels to decrease at the same rate. And that's indeed what we observe (Figure 1).
Figure 1: Atmospheric Oxygen Concentration observed from Cape Grim, Tasmania
There's no reason to expect that a natural release of CO2 would have any effect on atmospheric O2 levels. On the other hand, the O2 concentration is changing exactly as we would expect from a fossil-fuel driven CO2 increase.
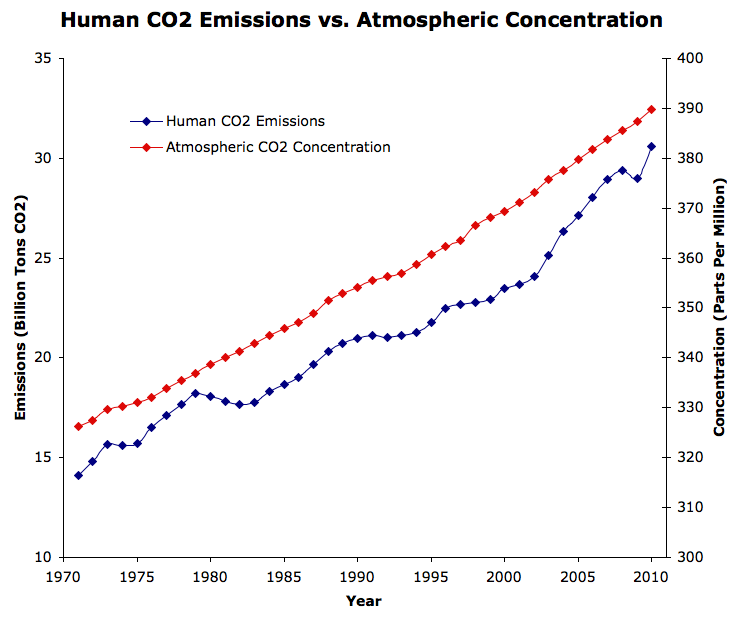
CO2 Rise is Smoother than Temperature
Some, most recently Murry Salby, have argued that the CO2 rise is in reponse to the temperature rise. However, the temperature rise has been quite erratic (because there are many factors which impact the average global temperature, especially in the short-term). If atmospheric CO2 changes were in response to temperature changes, then we would expect to see an erratic rise in CO2 as well. Instead, the atmospheric CO2 increase is very smooth, similar to the increase in human CO2 emissions.
Figure 2: Human CO2 emissions (blue, left y-axis, Source: IEA) vs. atmospheric CO2 concentration (red, right y-axis, Source: Mauna Loa record)
Isotopic Signature
Carbon is composed of three different isotopes: carbon-12, 13, and 14. Carbon-12 is by far the most common, while carbon-13 is about 1% of the total, and carbon-14 accounts for only about 1 in 1 trillion carbon atoms in the atmosphere.
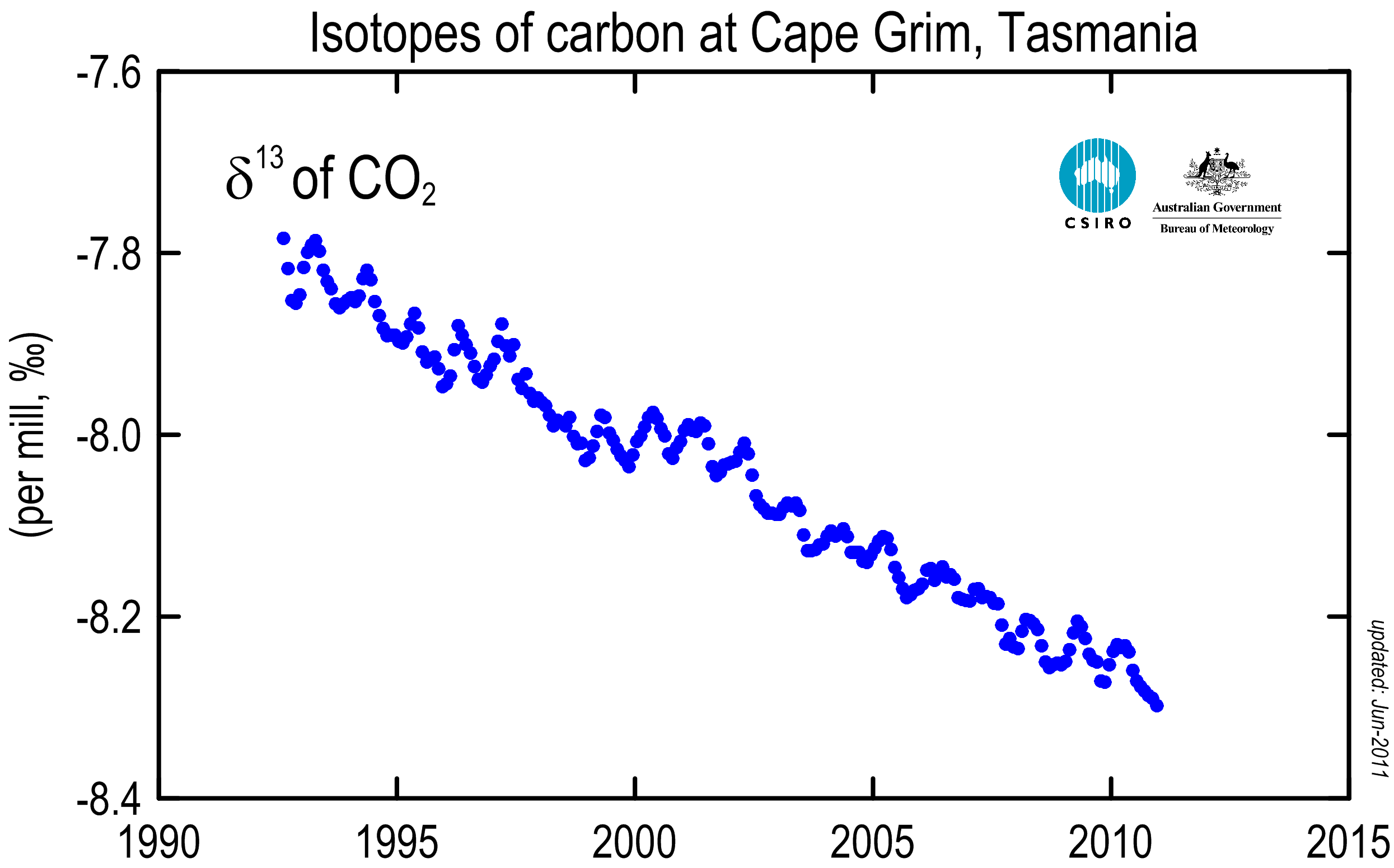
CO2 produced from burning fossil fuels or burning forests has a different isotopic composition from CO2 in the atmosphere, because plants have a preference for the lighter isotopes (carbon-12 and 13); thus they have lower carbon-13 and 14 to 12 ratios. Since fossil fuels are ultimately derived from ancient plants, plants and fossil fuels all have roughly the same carbon-13 to 12 ratio – about 2% lower than that of the atmosphere. As CO2 from these materials is released into, and mixes with, the atmosphere, the average carbon-13 to 12 ratio of the atmosphere decreases.
Reconstructions of atmospheric carbon isotope ratios from various proxy sources have determined that at no time in the last 10,000 years are the carbon-13 to 12 ratios in the atmosphere as low as they are today. Furthermore, the carbon-13 to 12 ratios begin to decline dramatically just as the CO2 starts to increase — around 1850 AD. This is exactly what we expect if the increased CO2 is in fact due to fossil fuel burning beginning in the Industrial Revolution.
Figure 3: Atmospheric carbon-13 ratio observations from Cape Grim, Tasmania
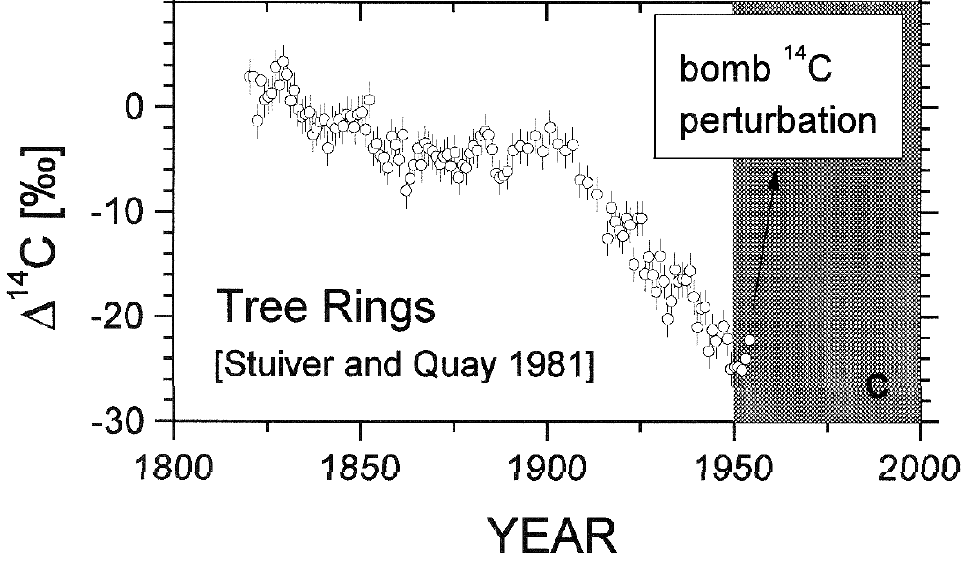
These isotopic observations confirm that the increase in atmospheric CO2 comes from biogenic carbon, not from the oceans or volcanoes. Some "skeptics" like Murry Salby argue that the carbon-13 ratio isn't unique to fossil fuels. However, because the carbon-14 ratio has also decreased significantly (Figure 4), we know it's from old (fossil fuel) sources, not modern sources. This is not new science either, it's something we've known for over half a century (Revelle and Suess 1957), and there have been many studies confirming these results. For example, Levin & Hesshaimer (2000):
"It has been erroneously argued that the observed atmospheric CO2 increase since the middle of the 19th century may be due to an ongoing natural perturbation of gross fluxes between the atmosphere, biosphere, and oceans. That the increase is in fact a predominantly anthropogenic disturbance, caused by accelerated release of CO2 from burning of fossil fuels, has been elegantly demonstrated through 14C analyses of tree rings from the last two centuries (Stuiver and Quay 1981; Suess 1955; Tans et al. 1979)."
Figure 4: Temporal change of carbon-14 ratio in tree rings grown at the Pacific coast (Levin & Hesshaimer 2000)
Settled Science
As you can see, there are many lines of evidence showing that the increase in atmospheric CO2 is due to human fossil fuel combustion. Each one of these lines of evidence is very conclusive on its own, and when all put together, it's abundantly clear that the science is settled on this issue.
As you can see from the snazzy new button created by John Cook at the top of this post, we've created a new series entitled 'Settled Science'. All too frequently we hear comments from "skeptics", like this one from Salby:
He said he had an “involuntary gag reflex” whenever someone said the “science was settled”.
“Anyone who thinks the science of this complex thing is settled is in Fantasia.”
There are some scientific issues for which the supporting evidence is so overwhelming and clear, that it's accurate to say the science is settled. The anthropogenic nature of the atmospheric CO2 increase is one of those settled issues, whether it makes Salby gag or not. In future posts in this series, we will investigate other issues for which the science is clearly settled.
This is the rebuttal to the myth CO2 increase is natural, not human-caused































 Arguments
Arguments






























Greetings, I'm new here. I was directed to Murry Salbys 'C-14 proof', apparently 'Control of atmospheric CO2 Part 1&2' in the obscure 'Journal' 'Science of Climate Change'.
Does anybody has any informations about where exactly he goes wrong in his proof?
EnderWiggin:
First, you've posted the same question in three locations. That is bad form. In the main menu under the masthead, the "Comments" link will show any new comments on any thread, so posting your question once will allow it to be found easily.
As for Salby, without a link to the papers I can't tell what that particular version of Salby's wanderings are wrong, but his name has been a frequent occurence here. You can use the search function at this site to find more, but in roughly chronoogical order here are several posts that discuss errors in Salby's work. I don't think he's come up with anything new in many years, so even old posts will probably cover any "recent" errors.
https://skepticalscience.com/Murry-Salby-CO2-rise-natural.htm
https://skepticalscience.com/Murry-Salby-Confused-About-The-Carbon-Cycle.html
https://skepticalscience.com/salby_correlation_conundrum.html
https://skepticalscience.com/salbyratio.html
[BL] Edited 2023/01/12/ to delete a link that pointed to an unpublished web page.
A follow-up to my comment @ 69, which was a response to EnderWiggin @ 68.
On Tuesday, I did a search for the title and author (Salby) that EnderWiggin provided. I was able to find parts 1 and 2 on a site hosted at scc.klimarealistene.com - but by Wednesday, that domain name had disappeared and could no longer be reached.
klimarealistene.com does still exist, but has no signs of the papers. A bit of searching on their web page found a link to scienceofclimatechange.org. Eventually, the two papers were found on this page (Volume 1.2 December 2021).
A bit of background. Klimarealistene is a well-known Norwegian climate "science" contrarian group. The "journal" Science of Climate Change is their creation. I suspect the change in web location has to do with reorganization of the journal's online pages. The old "scc" portion of the klimarealistene link was undoubtedly short for Science of Climate Change.
On the main SCC page, they say:
Thanks alot to Bob for his answers! I already knew the four articles on skepticalscience you linked to, but apparently the C-14-articles were not adressed therein.
Sorry for posting several times, I just thought to increase my chance to get an answer. Next time, I'm going to post only once.
The C-14 article is just lipstick on an old pig - the same errors that permeate earlier papers are applied to C-14 concentrations instead of just CO2.
The key factor is that differentiation over the short term cannot tell you much about long-term processes. There is a fundamental error, covered in the "correlation" link above, but not really expanded on or explicitly stated. Basically, when you differentiate (look at the short-term rates of variation) you eliminate the constant terms. Many moons ago I expressed this in a private forum as follows:
Salby and Harde forget that integration is not complete without adding a constant - a moot point if you are just doing the integration symbolically, but absolutely critical if you want to put actual numbers on it. CO2 does track the integral of temperature - as long as you don't forget to add back in the constant that dominates the correlation. Without the constant, there is no correlation, which tells us that the short-term variations in temperature are not affecting the long term buildup of CO2. Because Salby sees a correlation between the noise in T and the noise in CO2, he mistakenly assumes that integration will entirely reverse the differentiation without reference to the constant.