SkS Analogy 15 - Ice Tea and Temperature Rise
Posted on 16 October 2018 by Evan, jg
Tag Line
Even on a hot day, as long as your glass of tea has ice it is a nice, cool drink. Only when the ice disappears does your drink start to warm up.
Elevator Statement
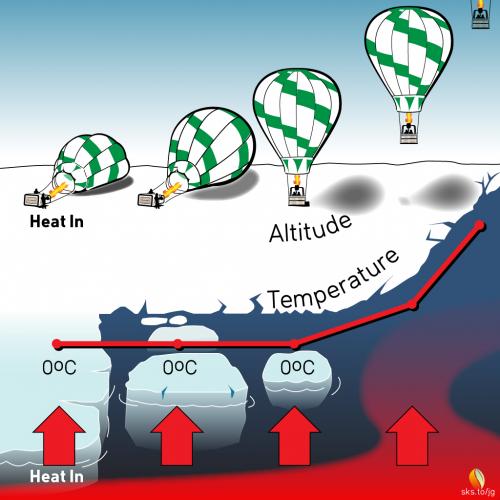
The height of a balloon above the ground is not proportional to the amount of hot air you put into the envelope.1 In fact, when you first start filling a balloon all that happens is that the hot air causes the envelope to lift off of the ground, applying tension to the basket and its occupants, but there is no upward motion of the balloon at that point. You pump in more and more hot air, and if select US Senators were standing by they would likely proclaim that there is absolutely no effect of hot air on the balloon.
The problem, of course, is that a huge amount of hot air must be injected into the balloon just to overcome the weight of the envelope, the basket, and its occupants. As long as the upward force is less than the downward, restraining force, nothing happens. But once the upward force overcomes the downward force, up you go, and any small addition of hot air2 at this point causes you to accelerate faster and faster skyward.
Ever wonder why you put ice cubes into your water and not cold rocks? The difference between ice and rocks is that the temperature of a cold rock will slowly increase along with the liquid it is trying to keep cold, and will do no better keeping the liquid cool than the cool liquid itself.
However, as long as there is ice in your glass, the combination of ice and tea will stay near 0°C. As soon as the ice melts and is completely gone the temperature of your tea starts to rise, and soon your drink becomes warm and tasteless. The reason for using ice is that the temperature of ice cannot rise above 0°C, whereas a cold rock will easily warm up past 0°C. If there is ice in your glass, it stabilizes the temperature to near 0°C. Read on in the next section to learn about the property of ice that keeps your ice tea near 0°C.
In the last 40 years we’ve lost about 70% of the Arctic ice volume.3 What will happen to the temperature of the arctic when the ice completely disappears?
Climate Science
If ice keeps your drink near 0°C, do you suppose ice has a similar effect of keeping the Arctic ocean at the North Pole near 0°C? What happens when the ice is gone and the oceans start to warm up past 0°C? Ice is like an anchor because as long as there is ice in the water, it keeps the temperature at reliably stable, cold temperatures. The equatorial regions of the Earth are at reliably warm temperatures. The difference between these two reliably consistent temperatures produce global winds (e.g., jet stream) that blow in consistent patterns. As Arctic ice disappears, the average Arctic temperature increases,4 and the stabilizing force anchoring the Earth’s large-scale winds (i.e., the jet stream) begins to waiver. Instead of having well-anchored, well-behaved winds, we inherit poorly-anchored winds that blow more erratically. This is part of the cause of the Polar Vortex dipping further down south than we’re used to. Weather patterns and hurricanes getting stuck and causing torrential rains may be linked to the jet stream slowing down and meandering more.
There is a special property of solids called the latent heat of fusion. The latent heat of fusion refers to the energy absorbed by a substance as it changes from a solid to a liquid. It’s a fancy term that can be easily illustrated as follows. Start with a chunk of ice at -10°C. Add a little heat and the temperature will quickly rise to 0°C, at which point the temperature stops rising. Ice made from pure water cannot exist at 1°C: only at 0°C and colder. At 0°C a phase change occurs, meaning that the solid ice starts to melt. It takes a lot of energy! Lots of energy to melt ice, and while the ice is melting, absorbing huge quantities of energy, the temperature of the ice does not change. This is one of the absolutely amazing properties of stuff as it changes from a solid to a liquid. In fact, ice absorbs so much energy while it’s melting, that the energy required to melt 1 kg of ice is enough to raise the temperature of 1 kg of water by 80°C!
Think of the difference between the energy needed to warm something up vs the energy to melt something (i.e., the latent heat of fusion) this way. You’re walking along and you come to a small hill. It takes you extra energy to walk up this small hill, but not that much extra. Maybe you huff and puff a bit and start to sweat, but overall you climb the small hill with no problem. This is similar to the energy needed to warm something up. Now imagine that someone strapped wings onto your arms and you try to fly. This is your “phase-change” event, in that you are going from being bound to the solid ground to being airborne. If you were strong enough to make the wings work, you would find that it takes a lot more energy to get yourself off the ground than it does to walk up the small hill. Assuming that you were able to get yourself airborne and keep yourself there, the extra energy it takes to rise up further into the air is small compared to the energy it took to get airborne in the first place. The point is that the energy to warm up a solid, a liquid, or a gas is small compared to the energy required to change a solid to a liquid (i.e., melting) or to change a liquid to a gas (i.e., evaporation). And even though it takes you lots and lots of energy to get airborne, your elevation does not change much at all. If you put that same energy into walking up a hill, you could climb very high up for the same amount of energy.
Part of the deceptive problem with global warming is that most people focus on air temperature only, and if it has not increased too much over a 10-year period, they say that global warming is not happening or that it is not too bad. What is missed in this analysis, however, is that the energy is still going into Earth’s system, but much of the energy is being used to melt ice or evaporate water (evaporation is another phase change from liquid to vapor that absorbs even more energy than required to melt ice). This ice melting continues until the global temperatures increase to a certain level, because the switch that stops global warming is radiation, which is a process that is controlled by temperature. With a given amount of GHGs in the atmosphere, heat will continue to be pumped into Earth’s system until the temperatures rise enough for radiation to shut down additional warming. Until that point we continue to melt ice, which is a slow process. But once ice in a particular area of the Earth is gone, the temperature of that area will rise much faster, because with the ice gone, the water that remains increases in temperature more quickly than when the ice was present.
Read here for more information about the relationship between temperature, the latent heat of fusion, and the energy of the atmosphere.
Footnotes
1. This is the part of the balloon that holds the hot air or helium.
2. Obviously you must continue adding some hot air to make up for the cooling of the gases.
3. See the NASA data set and this video animation of sea-ice volume decrease.
4. Because the Arctic is a large area, the temperature will only be moderated by the ice where ice remains. Where ice has already melted the temperature is not mitigated by the effect of the ice and the temperature will rise. Therefore, the idea is not that the temperature of the entire Arctic will remain near 0°C as long as there is some ice remaining, because areas that have become ice free will begin to warm more rapidly, without the restraining effect of ice. The actual physics is more complicated and depends on Ocean and Atmospheric currents, but the general idea is similar to what happens in a glass of ice tea.
5. This discussion is for pure water at atmospheric pressure. For salt water the freezing point is a bit colder, near -2°C, and an equal mixture of ice, water, and ammonium chloride freezes at -18°C, which is used to define the 0 point of the Fahrenheit scale.































 Arguments
Arguments






























Perhaps the title could be revised to “Iced Tea and Temperature Rise - Tipping Points”, with the term 'tipping points' mentioned regarding the phase transition examples that are presented (balloon starting to lift-off or, finally getting air-borne using wings).
Adding enough CO2 to the atmosphere to result in the elimination of year-round ice in any region is a tipping point for that region. And that CO2 level will be reached before the actual observation of the end to year-round ice. A related concern that could be added in the section discussing how regions without year-round ice will warm more rapidly is: When glaciers completely melt before the end of a summer season they will fail to deliver water downstream through an entire summer season, which can be a disastrous result that will suddenly occur at some time after the tipping-point level of CO2 has been forced into the atmosphere.
Another concern could be added regarding reduction of arctic ice extent. There is increased solar energy absorbed in the Arctic waters that are no longer covered by ice during the northern hemisphere summer, that time of the year when the sun shines on the Arctic. That amplifies the concern about the amount of warming of Arctic waters. Arctic waters that are not near surface ice will get warmer than waters near or under sea ice.
I think that the part about rocks in a drink is not a great example for this very relevant technical point. Many cold things, other than ice successfully help keep beverages cold:
Also, a cold rock could do a lot of beverage chilling. The lower the initial temperature of the rock the more cooling it can do. The disadvantage of a cold rock in a drink, other than it potentially being dirty in its many crevices, is that it will be at the bottom of the drink. A floating cooling element is better. As the drink warms up the warmer contents rise to top. With a rock in the bottom, each sip from the top will be warm unless the drink is stirred before each sip.
Other people may come up with points like that which would be a distraction from the validity of the technical points being made.
Agree that a really cold rock would work to cool a drink. I should have written that if you place a rock at 0C into a drink, it will warm up with the drink and do nothing to keep the drink cool, whereas if you place ice at 0C into a drink, it will keep it cool while it melts. That is, the main point is that during a melting substance will keep the surrounding liquid cool because it absorbs heat while it melts.
At the Arctic Blogspot they have been talking about this for a long time. Over there it's fearmongering. Here it's science. As for "One Planet's" comment - there is no cold mug to put the Arctic Ocean into. There are no cold rods to insert into it. And we are not going to refreeze it using frozen plastic balls or rocks from the freezer. So I fail to see any relevance to your points...
But back to the article - doesn't this seem in contradiction to the IPCC notion that the Arctic Ocean can go ice-free, but then re-freeze magically and not be ice-free again for another 9 or 99 years. That is what the IPCC claims! In a warming world, with surges of warm water into the Arctic becoming more frequent, they think this is possible, even likely. Amazing.
Science is all about looking at all possibilities. Even the watered-down IPCC report says that the human race is going to be in big trouble in less than 20 years. The Arctic Blogspot says we are in worse trouble sooner than that. Yet the IPCC, which tries to strike an acceptable balance between science and political reality, is considered pure science, while the latter is dismissed as hysterical. And that dismissal is not science. It just isn't.
Oh, and for those who believe the future temp rise will be linear - do you really think that it will happen that way when this, and other, feedbacks start to seriously bite? Because that conclusion would also fly in the face of long-established science. Nature isn't big on linear change. She loves those exponentials! Ask Albert Bartlett...
Interestingly, the latent heat of evaporation is 540 calories per gram or over 6 times as much as the latent heat of melting. If warm moist air flows from the Arctic Ocean over Greenland, the heat from one gram of water vapor condensing on the ice will give up enough heat to melt over 6 grams of ice. This is in addition to whatever 'sensible heat' the air contains.
Excellent article and analogies, but surely if you add cold stones to a glass of wine, heat energy will flow from the wine to the colder stones so cooling the wine? Obviously the stones warm up fast so the effect is much more limited than ice.
Sunspot @3, I would agree that the IPCC are a little too conservative or understated, but not to the extent you and the arctic news website claim. The difference is important, because wild claims attacking the IPCC will undermine trust in it.
I have just had a read of your arctic news website including the 12 points where it claims the IPCC is misleading. I just think most of this is wrong. Theres no obvious acceleration thus far in global temperatures in recent decades, if you look at the hadcrut or nasa data, although the IPCC project there will be in coming decades and with good reason. The IPCC does consider all the things you claim it doesn't consider, such as the water vapour feedback and methane clathrates, but doesn't reach your conclusions. It doesn't see this as leading to quote your website to " a potential global temperature rise (from 1750) of more than 10ºC by 2026, as illustrated in the image at the top."
Its also absurd of this website to claim the IPCC understate the problem for humanity, given the strident wording of this latest report on 1.5 degrees - and its good to see a sense of urgency in this report.
This is not to suggest the IPCC are perfect. This media article from the Guardian claims evidence that the summary for policy makers gets watered down, for example language gets changed from highly likely to likely etcetera. This would not be surprising as this document is signed off by politicians and bureaucrats from sceptical countries, but there's no evidence that the detailed science is watered down. Just consider this: The IPCC projet that if we continue to burn fossil fuels global temperatures could possibly hit over 10 degrees celsius by the year 2300. This would be totally catastrophic and should be enough to scare the pants off anyone with a functioning brain! So this is hardly the IPCC playing risks down.
There are recently emerging concerns and evidence about melting permafrost and some excellent science on the history of hothouse earth and various tipping points here in this article, that are cause for considerable concern, but this is new material, and so clearly wasn't in the last IPCC report. I would expect it to be in the next report and would be very concerned if it wasn't highlighted.
nigelj@5, if you put the stones it at the same temperature as the wine it will be no more effective at keeping the wine cool than replacing the stones with wine. If the stones are put in colder than the wine, then they will cool the wine. The real point, of course, is that a phase change (from solid to liquid) soaks up a lot of energy without the temperature increasing. The temperature of rocks will always increase as they soak up heat.
Sorry if I am confusing the issue by using rocks. I will put them back in my head. :-)