The human fingerprint in coral
Posted on 11 October 2010 by John Cook
Atmospheric carbon dioxide has risen by nearly 40% since pre-industrial times. Of course, all this extra CO2 coinciding with our dumping of billions of tonnes of CO2 into the air might be pure coincidence. How can we be sure we're responsible? Extra evidence comes from the different types of carbon isotopes found in the air. The most common carbon isotope is carbon-12 (12C) which is found in roughly 99% of the carbon dioxide in the atmosphere. The slightly heavier carbon-13 (13C) makes up most of the rest. Plants prefer carbon-12 over carbon-13. This means the ratio of carbon-13 to carbon-12 is less in plants than it is in the atmosphere. As fossil fuels originally come from plants, it means when we burn fossil fuels, we're releasing more 12C into the atmosphere. If fossil fuel burning is responsible for the rise in atmospheric CO2 levels, we should be seeing the ratio of 13C to 12C decrease.
That is exactly what we observe. Atmospheric measurements of carbon dioxide find the ratio of 13C to12C (otherwise refered to ?13C) has been steadily decreasing over the last few decades (Ghosh 2003). However, this data only goes back to the 1980s. Fortunately, corals provide a window further into the past. In Evidence for ocean acidification in the Great Barrier Reef of Australia (Wei et al 2009), the authors drilled a coral core from Arlington Reef, situated in the middle of the Great Barrier Reef. This enabled them to measure ?13C going back to 1800.
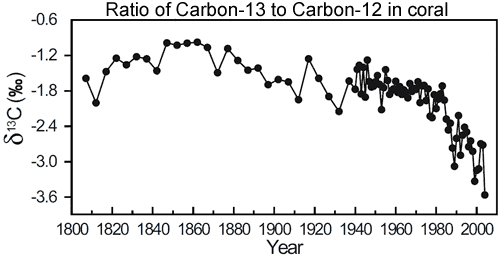
Figure 1: Change in ?13C (13C/12C ratio) in coral from Arlington Reef (in the centre of the Great Barrier Reef).
What they find is the ratio of carbon-13 to carbon-12 is relatively steady over much of the last two centuries. However, it starts to dramatically decrease in the latter half of the 20th Century. Increasing anthropogenic emissions of CO2 not only increased the levels of atmospheric CO2 concentration but also decreased the ?13C composition of the atmosphere. Thus, the decrease in ?13C is attributed to the burning of fossil fuels.
The story doesn't end there. As CO2 increases in ocean waters, seawater pH levels fall. Some key marine organisms, such as calcareous micro-organisms and corals, have difficulty maintaining their external carbonate skeletons when pH levels drop. The coral core extracted from Arlington Reef also provided measurements of boron isotope levels (?11B), which act as a proxy for seawater pH. They found that from 1941 to 2004, there was an overall trend of decreasing ?11B which corresponds to a drop in pH levels. The positive correlation between coral ?11B and ?13C provides strong confirmation that seawater acidification is closely linked to the anthropogenic CO2 emissions from burning of fossil fuels.
Thus fossil fuel burning is a cause of both global warming (which causes coral bleaching) and ocean acidification. To add further insult to injury, the paper also finds that coral bleaching may have the effect of further reducing pH levels. It seems coral reefs just can't catch a break.































 Arguments
Arguments
























 0
0  0
0






Comments