 Arguments
Arguments
 Software
Software
 Resources
Comments
Resources
Comments
 The Consensus Project
The Consensus Project
 Translations
Translations
 About
Support
About
Support


Latest Posts
- Skeptical Science New Research for Week #16 2025
- Climate Adam: Climate Scientist Reacts to Elon Musk
- Sabin 33 #24 - Is wind power too expensive?
- EGU2025 - Picking and chosing sessions to attend on site in Vienna
- 2025 SkS Weekly Climate Change & Global Warming News Roundup #15
- Fact brief - Is the sun responsible for global warming?
- Skeptical Science New Research for Week #15 2025
- Renewables allow us to pay less, not twice
- Sabin 33 #23 - How much land is used for wind turbines?
- Our MOOC Denial101x has run its course
- 2025 SkS Weekly Climate Change & Global Warming News Roundup #14
- Fact brief - Is Mars warming?
- Skeptical Science New Research for Week #14 2025
- Two-part webinar about the scientific consensus on human-caused global warming
- Sabin 33 #22 - How does waste from wind turbines compare to waste from fossil fuel use?
- Clean energy generates major economic benefits, especially in red states
- 2025 SkS Weekly Climate Change & Global Warming News Roundup #13
- Skeptical Science New Research for Week #13 2025
- Climate skeptics have new favorite graph; it shows the opposite of what they claim
- Sabin 33 #21 - How does production of wind turbine components compare with burning fossil fuels?
- China will need 10,000GW of wind and solar by 2060
- 2025 SkS Weekly Climate Change & Global Warming News Roundup #12
- Skeptical Science New Research for Week #12 2025
- Climate Fresk - a neat way to make the complexity of climate change less puzzling
- Sabin 33 #20 - Is offshore wind development harmful to whales and other marine life?
- Do Americans really want urban sprawl?
- 2025 SkS Weekly Climate Change & Global Warming News Roundup #11
- Fact brief - Is waste heat from industrial activity the reason the planet is warming?
- Skeptical Science New Research for Week #11 2025
- Visualizing daily global temperatures
Archived Rebuttal
This is the archived Intermediate rebuttal to the climate myth "Ocean acidification isn't serious". Click here to view the latest rebuttal.
What the science says...
|
The current debate on the connection between CO2 emissions and climate change has largely overlooked an independent and equally serious problem, the increasing acidity of our oceans. Last December, the re |
The current debate on the connection between CO2 emissions and climate change has largely overlooked an independent and equally serious problem, the increasing acidity of our oceans. Last December, the respected journal “Oceanography” published projections (see graphic below) for this rising acidity, measured by falling pH [i], through to the end of the century [ii]. In 2095, the projected average ocean surface pH is 7.8, and lower still in the Arctic Ocean.
![]()
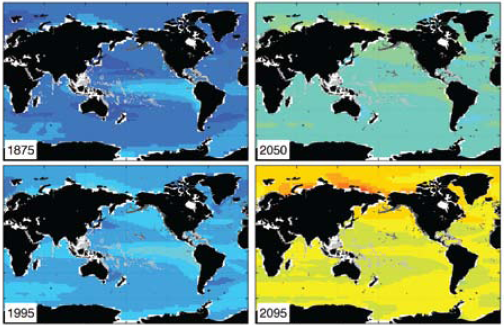
Fig 1: Ocean surface pH - historical values and projected future values based on current emission projections.
![]()
CO2 in the atmosphere has increased from 278 ppm in pre-industrial times to 390 ppm today. During this time, the amount of CO2 dissolved in the ocean has risen by more than 30% [iii], decreasing the pH of the ocean by 0.11 units. As with CO2 and global warming, there is some lag between cause and effect. That means that, even if all carbon emissions stopped today, we are committed to a further drop of up to 0.1 units.
The close relationship between CO2 in the atmosphere, CO2 dissolved in the ocean, and the effect of the latter in falling pH, is illustrated by the graph [iv] below:
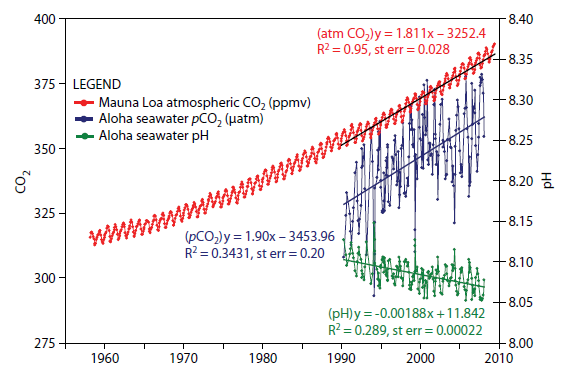
Fig 2: Annual variations in atmospheric CO2, oceanic CO2, and ocean surface pH. Strong trend lines for rising CO2 and falling pH.
CO2 dissolves in waterto form carbonic acid. (It is worth noting that carbonic acid is what eats out limestone caves from our mountains.) In the oceans, carbonic acid releases hydrogen ions (H+), reducing pH, and bicarbonate ions (HCO3-).
CO2 + H2O => H+ +HCO3- (1)
The additional hydrogen ions released by carbonic acid bind to carbonate ions (CO32-), forming additional HCO3-.
H+ + CO32- => HCO3- (2)
This reduces the concentration of CO32-, making it harder for marine creatures to take up CO32- to form the calcium carbonate needed to build their exoskeletons.
Ca2+ + CO32- => CaCO3 (3)
The two main forms of calcium carbonate used by marine creatures are calcite and aragonite. Decreasing the amount of carbonate ions in the water makes conditions more difficult for both calcite users (phytoplankton, foraminifera and coccolithophore algae), and aragonite users (corals, shellfish, pteropods and heteropods).
The photo below left shows healthy specimens of calcifying phytoplankton Gephyrocapsa oceanica. The photo below right shows the damage to the same creature under conditions expected by the end of the century.
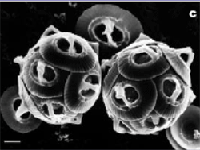
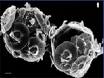
Fig 3: Healthy phytoplankton; same species with malformed shell plates as a result of damage by seawater with simulated end of century chemistry.
Source: Nature, Reduced Calcification of Marine Phytoplankton in Response to Increased Atmospheric CO2, Issue 407 p.364 -367
It is often said that a picture is worth a thousand words.
Research in the Southern Ocean provides evidence that the formation of foraminifera shells is already being affected. Even though these creatures use calcite, which is less soluble than aragonite, there are already clear signs of physical damage. According to Dr. Will Howard of the Antarctic Climate and Ecosystems Cooperative Research Centre in Hobart, shells of one species of foraminifera (Globigerina bulloides) are 30 to 35 percent thinner than shells formed prior to the industrial period.[vi]. The photo below left shows a pre-industrial exoskeleton of this species obtained from sea-floor sediment. The photo below right shows a exoskeleton of a live specimen of the same species obtained from the water column in the same area in 2007. These stunning images were obtained using an electron microscope. (An interview with Dr. Howard was broadcast on the Catalyst television program). [vii] What is staggering is the amount of erosion in the right image compared to the left. The right sample look porous with larger holes and a 10-fold increase in their number. These and creatures like them are at the base of an ocean food chain, and they are already seriously damaged. If they are lost, it is not just biodiversity we are losing, but our food supply as well.
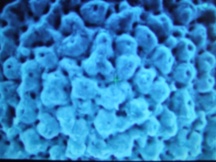
Fig 4. Pre-industrial and current samples of Globigerina bulloides from same location. Latter shows extensive erosion with a ten-fold increase in holes.
Source: Australian Broadcasting Corporation, Ocean Acidification – The Big Global Warming Story, 13 September 2007
The implications of all of this are disturbing. For corals to absorb aragonite from seawater, the latter needs to be saturated in this mineral.
Now a report from NOAA scientists found large quantities of water undersaturated in aragoniteare already upwelling close to the Pacific continental shelf from Vancouver to northern California [v]. Although the study only dealt with the area, the authors suggest that other shelf areas may be experiencing similar effects.
 For corals like those in Australia’s Great Barrier Reef, the outlook is grim. They are threatened with destruction on two fronts, both caused by CO2 emissions. Not only do increased ocean temperatures bleach coral by forcing them to expel the algae which supplies them with energy (see photo at left) [viii], but increased ocean CO2 reduces the availability of aragonite from which reefs are made.
For corals like those in Australia’s Great Barrier Reef, the outlook is grim. They are threatened with destruction on two fronts, both caused by CO2 emissions. Not only do increased ocean temperatures bleach coral by forcing them to expel the algae which supplies them with energy (see photo at left) [viii], but increased ocean CO2 reduces the availability of aragonite from which reefs are made.
It is time to wake up. Our planet is dying. I urge you to find the time to view a 20 minute documentary on the problem of ocean acidification produced by the international Natural Resource Defence Council. Simply go to: www.acidtestmovie.com
Fig 5. Coral killed by above average ocean temperatures.
References and Notes
Intermediate rebuttal written by alan_marshall
Update July 2015:
Here is a related lecture-video from Denial101x - Making Sense of Climate Science Denial
Additional videos from the MOOC
Expert interview with Annamieke Van De Heuvel
Expert interview with Charlie Veron
Expert interview with Ove Hoegh-Guldberg
Updated on 2015-07-08 by pattimer.
THE ESCALATOR

(free to republish)
























































