How do human CO2 emissions compare to natural CO2 emissions?
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
| |||
|
The natural cycle adds and removes CO2 to keep a balance; humans add extra CO2 without removing any. |
|||||
Climate Myth...
Human CO2 is a tiny % of CO2 emissions
“The oceans contain 37,400 billion tons (GT) of suspended carbon, land biomass has 2000-3000 GT. The atpmosphere contains 720 billion tons of CO2 and humans contribute only 6 GT additional load on this balance. The oceans, land and atpmosphere exchange CO2 continuously so the additional load by humans is incredibly small. A small shift in the balance between oceans and air would cause a CO2 much more severe rise than anything we could produce.” (Jeff Id)
At a glance
Have you heard of Earth's carbon cycle? Not everyone has, but it's one of the most important features of our planet. It involves the movement of carbon through life, the air, the oceans, soils and rocks. The carbon cycle is constant, eternal and everywhere. It's also a vital temperature control-mechanism.
There are two key components to the carbon cycle, a fast part and a slow part. The fast carbon cycle involves the seasonal movement of carbon through the air, life and shallow waters. A significant amount of carbon dioxide is exchanged between the atmosphere and oceans every year, but the fast carbon cycle's most important participants are plants. Many plants take in carbon dioxide for photosynthesis in the growing season then return the CO2 back to the atmosphere during the winter, when foliage dies and decays.
As a consequence of the role of plants, a very noticeable feature of the fast carbon cycle is that it causes carbon dioxide levels to fluctuate in a regular, seasonal pattern. It's like a heartbeat, the pulse of the Northern Hemisphere's growing season. That's where more of Earth's land surface is situated. In the Northern Hemisphere winter, many plants are either dead or dormant and carbon dioxide levels rise. The reverse happens in the spring and early summer when the growing season is at its height.
In this way, despite the vast amounts of carbon involved, a kind of seasonal balance is preserved. Those seasonal plant-based peaks and troughs and air-water exchanges cancel each other out. Well, that used to be the case. Due to that seasonal balance, annual changes in carbon dioxide levels form regular, symmetric wobbles on an upward slope. The upward slope represents our addition of carbon dioxide to the atmosphere through fossil fuel burning.
Fossil fuels are geological carbon reservoirs. As such, they are part of the slow carbon cycle. The slow carbon cycle takes place over geological time-scales so normally it's not noticeable on a day to day basis. In the slow carbon cycle, carbon is released by geological processes such as volcanism. It is also locked up long-term in reservoirs like the oceans, limestone, coal, oil or gas. For example, the "37,400 billion tons of 'suspended' carbon" referred to in the myth at the top of this page is in fact dissolved inorganic carbon in the deep oceans.
Globally, the mixing of the deep ocean waters and those nearer the surface is a slow business. It takes place over many thousands of years. As a consequence, 75% of all carbon attributable to the emissions of the industrial age remains in the upper 1,000 m of the oceans. It has not had time to mix yet.
Fluctuations in Earth's slow carbon cycle are the regulating mechanism of the greenhouse effect. The slow carbon cycle therefore acts as a planetary thermostat, a control-knob that regulates global temperatures over millions of years.
Now, imagine the following scenario. You come across an unfamiliar item of machinery that performs a vital role, for example life support in a hospital. It has a complicated control panel of knobs and dials. Do you think it is a good idea to start randomly turning the knobs this way and that, to see what happens? No. Yet that is precisely what we are doing by burning Earth's fossil fuel reserves. We are tinkering with the controls of Earth's slow carbon cycle, mostly without knowing what the knobs do - and that is despite over a century of science informing us precisely what will happen.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
Before the industrial revolution, the CO2 content in the air remained quite steady for thousands of years. Natural CO2 is not static, however. It is generated by a range of natural processes, and absorbed by others. The carbon cycle is the cover-all term for these processes. It has both fast and slow components.
In the fast carbon cycle, natural land and ocean carbon remains roughly in balance and has done so for a long time. We know this because we can measure historic levels of CO2 in the atmosphere both directly, in ice cores and indirectly, through proxies. It's a seasonal response to things like plant growth and decay.
In stark contrast to the fast carbon cycle, the slow version operates over geological time-scales. It has affected carbon dioxide levels and therefore temperatures throughout Earth's history. The reason why the slow carbon cycle is so important is because many of the processes that lead to long-term changes in carbon dioxide levels are geological in nature. They take place over very long periods and do so on an erratic basis. The evolution of a species that has deliberately disturbed the slow carbon cycle is another such erratic event.
Annually, up to a few hundred million tonnes of carbon pass through the slow carbon cycle, due to natural processes such as volcanicity. That's small compared to the fast carbon cycle, through which some 600 billion tonnes of CO2 pass to-and-fro annually (fig. 1). However, remember that the fast carbon cycle is a give-and-take seasonal process. The slow carbon cycle instead runs in one direction or another over periods typically measured in millions of years.
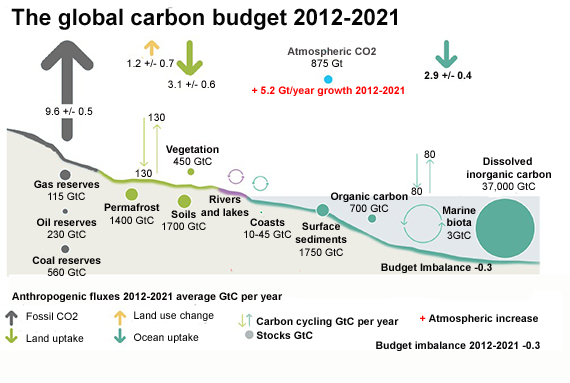
Fig. 1: Schematic representation of the overall perturbation of the global carbon cycle caused by anthropogenic activities averaged globally for the decade 2012–2021. See legends for the corresponding arrows and units. The uncertainty in the atmospheric CO2 growth rate is very small (±0.02 GtC yr−1) and is neglected for the figure. The anthropogenic perturbation occurs on top of an active carbon cycle, with fluxes and stocks represented in the background. Adapted from Friedlingstein et al. 2022.
Through a series of chemical and geological processes, carbon typically takes millions of years to move between rocks, soil, ocean, and atmosphere in the slow carbon cycle. Because of these geological time-scales, however, the overall amount of carbon involved is colossal. Now consider what happens when more CO2 is released from the slow carbon cycle – by digging up, extracting and burning carbon from one of its long-term reservoirs, the fossil fuels. Although our emissions of 44.25 billion tons of CO2 (in 2019 - source: IPCC AR6 Working Group 3 Technical Summary 2022) is less than the 600 billion tons moving through the fast carbon cycle each year, it adds up because the land and ocean cannot absorb all of the extra emitted CO2: about 40% of it remains free.
Human CO2 emissions therefore upset the natural balance of the carbon cycle. Man-made CO2 in the atmosphere has increased by 50% since the pre-industrial era, creating an artificial forcing of global temperatures which is warming the planet. While fossil-fuel derived CO2 is a small component of the global carbon cycle, the extra CO2 is cumulative because natural carbon exchange cannot absorb all the additional CO2. As a consequence of those emissions, atmospheric CO2 has accumulated to its highest level in as much as 15 to 20 million years (Tripati et al. 2009). This is what happens when the slow carbon cycle gets disturbed.
This look at the slow carbon cycle is by necessity brief, but the key take-home is that we have deeply disturbed it through breaking into one of its important carbon reservoirs. We've additionally clobbered limestones for cement production, too. In doing these things, we have awoken a sleeping giant. What must be done to persuade us that it needs to be put back to sleep?
Cartoon summary to counter the myth

This Cranky Uncle cartoon depicts the "Cherry picking” fallacy for which the climate myth "Human CO2 emissions are small" is a prime example. It involves carefully selecting data that appear to confirm one position while ignoring other data that contradicts that position. Source: Cranky Uncle vs. Climate Change by John Cook. Please note that this cartoon is illustrative in nature and that the numbers shown are a few years old.
Last updated on 17 September 2023 by John Mason. View Archives































 Arguments
Arguments








































YubeDude @250, there is not one isotope, but two.
First, C14 is a radioactive isotope with a short half life (5730 years) as a result of which C14 is effectively undetectable in carbon sources more than 50 thousand years old (at which stage it has fallen to 0.2% of its original value. Because of that, fossil fuels are almost completely devoid of C14, as are volcanic emissions. The very rapid decline in C14 in the atmosphere since the onset of large scale combustion of fossil fuels shows that the source of the rapid rise in atmospheric CO2 is devoid of C14, and therefore does not come from recent vegetation, or the ocean.
Second is C13. C13 is a stable isotope of carbon, that does not increase or decrease in quantity with age. It is heavier than C12, however, as a result of which many plants will take up proportionally more CO2 with a C12 isotope than with a C13 isotope. The result is that carbon from organic matter, including fossilized organic matter in the form of fossil fuels, is deficient in C13. The decline in the C13/C12 ratio in the atmosphere since the large scale combustion of fossil fuels shows the resulting increase in atmospheric CO2 to come primarilly from an (originally) organic source, ie, from vegetation or from fossil fuels.
Taking the information from both isotopes, we see that the increased CO2 cannot come from modern vegetation because of the decreasing C14 concentration, and that it also cannot have come from volcanoes because the decreasing C13/C12 ratio. The only possible source that explains both trends is fossil fuels. Ergo, the increased CO2 concentration is a result of the combustion of fossil fuels.
TY Tom for the reply. I understand about the carbon ratio and the meaning what I am interested in is if CO2 also caries the O-18 marker that would link that particular molecule of CO2 directly to combustion and not just a sink release or another anthropogenic use of older carbon sources such as chemical manufacturing that is petro-chemically based.
I am wondering if O-18 has any merit whitin the topic as I see no mention or research that attempts to link or associate its presence with A-CO2.
No quibble on my part as to the evidence rather just a currisoity in regards to this darn O-18. Thanks in advace for any light you may be able to shed.
YubeDude @252, I am not aware of any use of O18 as a marker of CO2 of organic origin. Of course, I am not expert in the field, so that only means it has not made it into popular presentations (or the IPCC reports). Further, searching google scholar does not readilly turn up studies using O18 as a marker for the organic origin of CO2.
I suspect it is possible in principle. However, fractionation of O18 in respiration is a confounding factor, and likely to be stronger. Further, atmospheric O18/O16 ratios will vary more with time than than C13/C12 ratios due to the fractionation of O18 in water by evaporation and condensation. No doubt there are other complexities of which I am not aware as well.
Sorry I can't help anymore.
I was wondering if somebody could explain a part of the answer to me? Fistly, the answer states that rises have been noticed. If this is true, why is the complete rise solely based on humans and why can this not be a dramatic rise naturally occuring? What is to say that this is a new rise not previously seen in history before that is natural? I do, think that humans contribute to a rise in co2....to clarify. Secondly, I am assuming that the things I heard on tv were true and that there is a hole in the ozone. If there is a hole, why wouldnt the co2 just escape through that hole? Why wouldnt oxygen and nitrogen escape through that too?
Im really just trying to get some answers here. The problem, from my perspective and I think a good majority of others, is that as a lot of the comments show, this is science. I am not a scientist. That doesnt mean I was raised to trust anybody telling me what they have is true. I need this explained is some everyday, simple language please.
Firstly, we know with reasonable accuracy how much coal and petroleum are being burnt every year. We know that not all of that stays in the atmosphere (or the rise in CO2 would be larger). At the moment, the sea is moping up some of those emissions and becoming less alkaline in the process (that is also measured) so CO2 is not coming from the sea. The change in the pH allows us to calculate how much CO2 is being dissolved. As the oceans warm, this will change and eventually the oceans will begin to emit CO2 - hopefully not in the next century or so.
Sources of CO2 have different ratios of the carbon isotopes C14, C13, C12. For instance, fossil fuels have no C14 (it is short-lived). You can look at ratios of these isotopes in the air and water and check if the proportions match emissions from humans or some other source.
The "hole in the ozone layer" is a pictureque but inaccurate description. All the gases (including ozone) in the atmosphere are bound to the earth by gravity. The ozone layers doesnt trap any gas. Ozone is produced in tiny amounts in upper atmosphere by interaction of oxygen with UV radiation. This has a very important effect in shielding the lower atmosphere (where we live) from UV. Chemicals released into the atmosphere (CFC) are chemically destroying ozone especially above Antarctic so that it is much thinner ("the hole") in those places.
rdbachel @254, you may be interested to read this summary of how we know the CO2 rise to be anthropogenic. The mass balance argument (we know how much we have emitted, and it is more than the increase in CO2) and the isotope arguments mentioned by scaddenp are the most important pieces of evidence, but no the only ones.
To slightly complicate things, had the temperature increase we experienced over the last century occurred without any increase in anthropogenic emissions over pre-industrial levels, we would still have experienced about 10% of the increase in CO2 levels that have actually occurred. This is known due to the known (from experiment) solubility of CO2 in water with temperature, and from comparisons to CO2 increases in past eras when temperatures have risen (as at the end of glacials). However, the rise in directly anthropogenic CO2 has been far faster than that potential increase. That is known from mass balance, and from the fact that ocean acidity increased over that period whereas it would have decreased if the CO2 increase in the atmosphere was due to thermal sources. Further, even if some of the increase should be attributed to temperature, that temperature increase is itself primarilly anthropogenic so that any CO2 increase in the atmosphere caused by it is also anthropogenic.
So called "skeptics" about AGW sometimes argue that the CO2 increase is natural and due to temperature rise. To do so they entirely ignore the rates at which CO2 rises in the atmosphere with increased global temperature (which are too small by a factor of 10 to account for the actual rise experienced), and the concurrent increase in ocean acidity, which proves the amount of CO2 dissolved in the ocean is increasing at the same time.
TC. I would have to humbly disagree with you on this one. I dont believe that we would have received a 10% increase - yet. Ocean mixing delays that solubility response.
scaddenp @257, based on ice core data, there is approximately a 90 ppmv rise in CO2 concentration between glacial and interglacial. That yields a CO2 increase per degree C between 11.25 and 30 ppmv per degree C with the former for an 8 C increase in temperature, and the later for an assume 3 C increase. A reasonable central estimate is 18 ppmv per degree C (5 degree increase). For the 0.8 C increase in temperature experienced since the industrial revolution, that yields and expected increase of 14.4 ppmv or 12% of the CO2 increase todate. As you say, it is unlikely that all of that increase would have happened over so short a time.
As an alternative emperical measure, taking the global temperatur anomaly from 1010 AD to 1800 AD (Mann 2008) and ice core CO2 records, the regression shows an 8.1 +/- 2.9 ppmv increase in CO2 per degree C. Based on that regression the expected increase for the global temperature increase since the pre-industrial of 0.8 C is 6.5 +/- 2.3 ppmv of CO2, or 3.5 - 7.3%. The time resolution of the CO2 record is 75 years, and I used a 75 year average of the temperature record to maintain equivalent resolution. That regression, therefore, yields a reasonable but not precise estimate of the increase that would have occurred from temperature increase alone.
As far as showing the absurdity of the pseudo-skeptic claims that the increase in atmospheric CO2 was caused by the temperature increase goes, the difference between 3% and 10% is not relevant. Therefore I indulged my habit of using conservative (for my position) estimates for rough figures where it makes little difference. The long and short, then, is that, yes I agree with you. But I don't think the difference is enough to warrant keeping track of for rough estimates.
Hi All,
I'm new here and hope, although this question is indirectly related, that it is not too far off topic to not be answered.
I understand how the oceans absorb c02 and the forces at play.
What I would like to know is how does c02 absorbed 'heat' from the atmosphere get into the ocean itself. What are the mechanisms at work that makes it possible for the ocean to be a 'heat sink' for atmospheric heat?
i.e "90% of the atmospheric heat ends up in the ocean" - Tim Flannery.
This assertion on face value appears to be in violation of the Laws of Thermodynamics.
i.e Two thermodynamic systems that are not in equilibrium with each other must have net sum energy transfer move in one direction only.
Because clouds are the observable evidence that net sum energy transfer occurs from the ocean to the atmosphere, and that net sum energy transfer between the atmosphere and the ocean can only be in one direction, how can the ocean be a heat sink for atmospheric heat as it would have to be a net sum value, which would be a violation of thermodynamics?
Any assistance here would be greatly appreciated.
Thanks
[DB] This post explains how increasing levels of CO2 heat the ocean. For continued questions on that mechanism, place those questions there, not here. Thanks!
Gac73,
I posted a response to your question here.
This landed in my mailbox a day or so ago. It comes from a gentleman whose speciality is Heliophysics. In addition to what he has to say here, I'd like to add that, the best I can tell, about .006, just over 1/2 of 1% of the atmosphere is made of CO2. And of that, depending on who you believe, the manmade portion of that amount is only 2%-7%. Either way, we're talking about an infinitesimal figure. Using the 7%, that still only comes to .000042 of the atmosphere is affected by manmade CO2 emmissions.
Color me stupid, but I just have a hard time believing that has any impact whatsoever.
Now, I do not claim in any way whatsoever to be a scientist. Those numbers are what I grabbed from a few of what I consider to be reliable internet sources. Please, if you have evidence to prove me wrong, by all means, do so. I'm open minded about science, and willing to listen to anyone who can provide substantive information. I think the main thing, which I've found from many sources, as the guy I'm quoting below points out, is that CO2 levels FOLLOW rising temps, not vice versa. Like I say, I just study as much as I can on something which interests me and try to make a sound conclusion based on what I find. And I am in no way offended by conflicting information. We'll just have to look deeper into the backgrounds of the sources to see if they have anything to gain or lose one way or the other.
"Currently the consensus is that 15% of global climate change is due to the sun. I think that this might be a bit low. for the last 10 years GISS [I assume he's referring to the Goddard Institute for Space Studies at Columbia University] has seen a decreasing, trend of global temperature. I would caution that the decrease is consistent with no change. CO2 continues to increase. Further the glacial record shows that increases in CO2 levels lag global temperature increase."
[JH] On this site, it's considered to be in poor taste to include more than one denier myth in a single comment. It's also in poor taste to try to disguise where you are coming from by quoting someone else.
Please note that posting comments here at SkS is a privilege, not a right. This privilege can be rescinded if the posting individual treats adherence to the Comments Policy as optional, rather than the mandatory condition of participating in this online forum.
Please take the time to review the policy and ensure future comments are in full compliance with it. Thanks for your understanding and compliance in this matter.
Common Sense > Brain Washed:
1) Benjamin Franklin said it best:
2) Your "common sense" requires not only that much of the public be brainwashed but that the vast majority of climate scientists have undertaken a conspiracy to delude the public. That is not common sense in any terms, but much closer to the ravings of a loon.
3) And for the moderator, if an offensive wanker (profanity removed) is allowed to use a monikor that amounts to an accusation of massive fraud by scientists, then he should be expected to take his lumps in turn.
4) I will be convinced that small concentrations are irrelevant when you sit in a chamber containing 400 ppm Arsenic pentaflouride (LC50 at 20 ppm) for an hour and tell me who little effect it has had.
(Warning: Just in case your sense is as sensible as your post suggests, LC50 means a 50% probability of death as a result of exposure to that concentration for an hour in test animals. Do not conduct this experiment.)
[JH] Your point #3 is spot on. I have also issued Standard Moderation Comment #1 on his/her post.
[PS] Please observe comments policy on profanity
Brain Washed wrote: "I'm open minded about science, and willing to listen to anyone who can provide substantive information."
You might have tried reading/searching first. The various arguments you present are all covered in the 'climate myths' section of this web-site;
"the best I can tell, about .006, just over 1/2 of 1% of the atmosphere is made of CO2"
No. The current atmospheric CO2 level is about 400 parts per million... 400 / 1,000,000 = 0.0004.
the manmade portion of that amount is only 2%-7% (BTW, [400 - 280] / 400 = 30%, whoever told you 2% to 7% is really bad at math)
Color me stupid, but I just have a hard time believing that has any impact whatsoever.
CO2 levels FOLLOW rising temps
"Currently the consensus is that 15% of global climate change is due to the sun."
P.S. Seriously, don't do Tom's hypothetical arsenic experiment.
CBDunkerson @263.
The 0.006 value being quoted is probably ppm CO2 by weight which is roughly 1.5x the 'by volume' value for dry air.
The 2%-7% is probably that derived from the relative size of CO2 fluxes into the atmosphere, from man-made sources and from natural sources, a particularly stupid value to use as the natural fluxes are bi-directional while the man-made ones only go one way.
MA Rodger @264, I believe the figure originally came from calculations of the residence time of CO2 in the atmosphere made from measurements of the rate at which the C14 spike from nuclear testing dissipated. From such measurements it was determined that approximately 203 gigatonnes of Carbon in the form of CO2 (GtC) leaves the atmosphere each year. Given a total atmospheric reservoir of 829 GtC, the average duration of a carbon atom in the atmosphere is just over four years. Ergo, for a year n years ago, the approximate fraction remaining in the atmosphere is 0.75^n. Even with an assumed constant emissions of 8 GtC per annum, that means only approx 24 GtC in the atmosphere was emitted from an anthropogenic source, and has never left the atmosphere since emission. That turns out to be 2.9% of the atmospheric concentration. (Obviously the figure will vary slightly with more exact calculation.)
Taking a simple ratio of annual gross natural emissions to annual anthropogenic emissions gives you a ratio of 4.1% if you only include fossil emissions, but 4.7% if you include emissions from land use change and 17.5% if you include all anthropogenic emissions including outgassing due to global warming.
Both calculations assume that any emissions from the ocean or plants and animals cannot have come from fossil sources. That is, of course, absurd. Indeed, it is assumed by both methods that most anthropogenic emissions are stored in either plants or the ocean as a result of the rapid exchange of CO2 between atmosphere and the other surface reservoirs of carbon. It follows that a certain proportion of the "natural emissions" are emissions of carbon that until a few hundred years ago (and in most cases a decade ago or less) was stored in a fossil reservoir. A rough calculation of the proportion of CO2 in the atmosphere that was recently in a fossil reservoir is then given by the ratio of the sizes of the combined reservoirs to the cumulative emissions. That works out to approximately 12% excluding the deep ocean and soil (for which exchange is slow). The actual number may be lower than that, but not by much. (It may also be higher given the average life span of trees.)
All this, however, is as you note, beside the point. The real question is how much of the increase in atmospheric emissions is due to anthropogenic emissions. To that the answer is 100%. Ergo, 30% of the current atmospheric concentration would not have been in the atmosphere without anthropogenic emissions. That is the case regardless of whether or not any individual atom was recently in a fossil reservoir for the approximately 20% of atmospheric CO2 that would not have been there without emissions, but whose carbon was not recently in a fossil reservoir has merely displaced CO2 whose carbon was recently in a fossil reservoir due to equilibrium exchanges.
(Note, all figures calculated using values from Fig 6.1 of AR5 WG1.)
Tom Curits @265
Any cursory look at two readily available data sets, Mauna Loa CO2 and global temperature by year, clearly shows that temperature is a strong driver in how much CO2 ends up in the atmosphere on a yearly basis. Note especially the 600% difference in 1992's and 1998's CO2, clearly based on temperature.
Also look at CO2 increase by year from 2000-2015, hardly changed at 2ppm per year despite a 68% increase in global emmisions over the same time period. Why isn't the rate of CO2 increase responding to human emissions? Because CO2 reponds far more to temperature, which has flatlined for 15 years.
[Rob P] - Surface warming still continues.....
And the last 16 years has seen a 50.3% increase in heat taken up by the Earth's climate system than the previous 16 years. Which means there's a lot more warming in the pipeline. The following image is from the IPCC AR5 WG1 on the oceans.
Rickeroo... A "cursory look" is not going to "clearly show" anything.
Another "cursory look" actually contradicts exactly what you're saying. If temperature were driving CO2 then why hasn't the atmospheric concentration of CO2 also "flatlined" over the past 15 years?
And Rickaroo, scientists aren't arguing from the correlation between the CO2 and temp graphs. Why would you? The physical mechanism of the greenhouse effect is extremely well-established--to the point of being instrumentally measured from the surface. If you want to make the argument you've claimed, you'll need to remove the greenhouse effect.
Temp does drive CO2, of course, because the warming oceans absorb less atmospheric C. The process is a feedback to initial and ongoing warming, though.
Rickaroo @266, I see what you mean. There is no correlation between CO2 concentrations and anthropogenic emissions at all, is there?
(Source)
Just out of curriousity, what is the short term correlation between CO2 concentration and temperature that you base your claims on?
DSL @268, the change in CO2 forcing from year to year is very small. Therefore the greenhouse effect does not explain the correlation between CO2 and temperature at subdecadal, or even decadal time scales*. Rather, warm water absorbs less CO2 than does cold water. Therefore in warm years, less CO2 is absorbed, while in cold years, more is absorbed - thus explaining much of the sub-decadal correlation. (Biological activity also explains some of it.) The key point, however, is that even in the warmest years, the increase in atmospheric CO2 is less than the amount of CO2 pumped into the atmosphere by anthropogenic emissions. Ergo that increase is explained by the anthropogenic emissions, with only variations around the mean increase explained by changes in Sea Surface Temperature. This is one of those areas of climate science supported by so much evidence that denial of it falls into the "flat earth society" level of intellectual analysis.
* On time scales of thirty plus years, however, it explains nearly all of it, in the last and current century.
There is also the yearly cycle of CO2 related to global vegetation, predominately the Northern Hemisphere which has more land (and hence vegetation) absorbing and releasing CO2 over the season. But that is a very small, short term, and essentially zero based variation, despite being a favorite graph of denialists.
[ I think that particular correlation and corresponding misleading graph, with long term CO2 growth and temperature changes being removed as (ahem) inconvenient facts, shows up at WUWT about once a month... ]
Rickeroo wrote: "Any cursory look at two readily available data sets, Mauna Loa CO2 and global temperature by year, clearly shows that temperature is a strong driver in how much CO2 ends up in the atmosphere on a yearly basis."
The correlation between temperature and the annual change in atmospheric CO2 is well known and has been since at least the work of Bacastow in the mid 1970s, and is largely due to the effect of ENSO on precipitation in the Americas, which in turn affects the uptake and release of CO2 by land vegetation (as KR mentions). This is explained in more detail in my article on Prof. Salby's misunderstanding of this correlation, where I show that a correlation with the annual increase has no mathematical relation to the cause of the long term rise (as the correlation is insensitive to the mean value of the annual increase, but it is the mean value that explains the long term rise. In particular, see the section "What does Mainstream Science say about all this?".
Some claim that this correlation is due to Henry's law, which suggests that the solubility of CO2 in the oceans depends on ocean temperature. However this neglects an important fact, which is that Henry's law also tells us that the solubility is proportional to the difference between the partial pressure of CO2 in the atmosphere and the concentration in the surface waters. Thus as atmospheric CO2 rises, its solubility in the oceans increases and the oceans take up CO2 in opposition to the rise in atmospheric CO2. It is the constant of proportionality that is sensitive to temperature. This is a good thing as it is a negative feedback that keeps the climate system more stable than it would otherwise be. So which factor dominates? The fact that atmospheric CO2 is rising more slowly than we are emitting CO2 into it shows that the natural environment as a whole is a net carbon sink, which tells us that the long term rise is being opposed by the natural environment, rather than being caused by it.
I am not sure what favourite WUWT graph KR refers to, but I imagine it looks something like this:
As you can see, there is a clear correlation between the CO2 records, and the temperature record. Of course, these are not the simple records. What I have done is to take each year from 1964-2009, and divided by the average of the eleven nearest years (inclusive). The purpose of doing that is that it eliminates any long term trend while retaining the annual variation. That is a good thing, because it allows me to take a regression of the two time series against each other, thereby determining the natural scale that maximizes similarity between the time series. In this case, that natural scale is 8.7, as indicated in the title. That is, for every 1 degree C increase in temperature, from this data we expect an 8.7 ppmv increase in CO2 concentration.
Put another way, based on the actual temperature and CO2 data, we expect the 1 C increase in Global Mean Surface Temperature (GMST) from 1910 to 2010 to have resulted in an 8.7 ppmv increase in CO2 concentration. As it happens, it has increased a little more than that.
Just using the GISS LOTI and Mauna Loa data used in constructing the graph, we can determine the approximate increase in CO2 from 1964 to 2009 was 67.95 ppmv (difference of eleven year means), while the temperature increase was 0.61 C. From that data, using the regression above, we can determine that just 5.29 ppmv of the increase was due to the increase in GMST, ie, just 7.8%.
However, there is still a strong correlation between temperature and CO2 over the period of Mauna Loa observations. Indeed, the correlation is 0.935 (RSQ = 0.874). That is not as good as the correlation between CO2 and cumulative emissions I mentioned @269 above. But it is still impressive. Very much better, for example than the 0.574 correlation (RSQ = 0.329) between the values once the trend is removed. That stronger correlation between the trend than the annual values tells us that, most probably, one is significantly responsible for the other. That is, either temperature is largely responsible for the CO2 trend; or CO2 is largely responsible for the temperature trend. However, we have excluded the former already with our regression. Ergo, the correlation between the annual data shows that the increase in CO2 is causing the increase in temperature (or at least, is largely responsible for it.)
It is no wonder Rickeroo (@266) invites us to have a "cursory look" at the data. If we only had a cursory look at the data, you might believe his interpretation of it. Once you analyze, it, however, you can see it conclusively refutes all of his claims.
Co2 from Man made drinks. Is co2 from say beer. A natural co2 emission? or a human emision. Or does it change after it is consumed. Some co2 is absorbed and expelled by the lungs. The remainder is belched back out?
DangerousDan @274, CO2 in beer and some softdrinks (ie, brewed Ginger Beer) comes from fermentation, and as such was originally drawn from the atmosphere by photosynthesis. Because they are originally drawn from the atmosphere, their return to the atmosphere merely completes the cycle. It does not increase atmospheric concentrations.
CO2 in carbonated drinks, however, may come from a variety of sources including fossil CO2, by cracking CO2 from the air by refrigeration, or from by products of other processes. The Coca Cola company, in particular, have stated that most of their CO2 used in drinks comes from by products of other processes, and hence do not constitute additional emissions to those other processes.
Finally, even if all the CO2 in soft drinks was additional emissions, it would constitute a tiny proportion of total emissions. Roy Spencer estimates total emissions from soft drinks as 1.46 million tonnes of CO2 per year. For comparison, the annual emission from fossil fuels 28.6 billion tonnes of CO2 per year. That is, soft drinks would contribute 0.005% of the problem if (contrary to fact) they used no recycled CO2.