Ocean acidification: global warming's evil twin
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
| |||
|
Ocean acidification threatens entire marine food chains. |
|||||
Climate Myth...
Ocean acidification isn't serious
'Our harmless emissions of trifling quantities of carbon dioxide cannot possibly acidify the oceans. Paper after paper after learned paper in the peer-reviewed literature makes that quite plain. Idso cites some 150 scientific sources, nearly all of them providing hard evidence, by measurement and experiment, that there is no basis for imagining that we can acidify the oceans to any extent large enough to be measured even by the most sensitive instruments.' (Christopher Monckton)
At-a-glance
Have you heard of ocean acidification? Does it mean that if you go swimming in the sea, you are liable to dissolve? No. You'll be OK because you are not a calcifying organism, such as a mollusc, a coral or a sea-urchin.
So why is ocean acidification serious? Because it can potentially lead to massive collapse of marine food-chains. Let's take a look at what the term means.
The pH scale, which measures acidity and alkalinity of water-based chemical solutions, runs from 0 (highly acidic) to 14 (highly alkaline), with pH 7 being the neutral halfway point. Importantly, the scale is logarithmic, meaning that a jump of one point towards zero means a tenfold increase in acidity.
Acidification simply means lowering the pH value from any point on the pH scale towards zero. It's similar to the way we talk about temperatures. If the pH of a solution shifts from 9 to 8, that is acidification, even though the pH is still on the alkaline side of neutral. Likewise, if the temperature rises from -40°C to -15°C, it has noticeably warmed, even though it's still darned cold.
Now, typical seawater is slightly alkaline at around pH 8.1. Rainwater, which always contains dissolved carbon dioxide (the old name for which was 'carbonic acid gas'), has a more acidic pH of around 5.6. You have likely visited or watched footage of spectacular caves, have you not? All carved out by carbonic acid, dissolving solid limestone over many thousands of years.
Carbonic acid is not only present dissolved in raindrops. It also forms by the dissolving of carbon dioxide at the air-water interface of our oceans. The more carbon dioxide in the air, the more goes into the oceans, driving their pH from 8.1 downwards. Now, the huge problem this creates, well before we get anywhere near the neutral value, is as follows.
Many marine organisms build and maintain their protective shells or skeletons from 'biogenic' calcium carbonate. The word biogenic means made by living things. These creatures extract the calcium and carbonate ions dissolved in seawater and combine them together. Under normal conditions, such calcium carbonate is stable in shallow waters. That's because dissolved carbonate ions are present in such high concentrations that the waters are said to be saturated with them.
But if seawater pH falls, even by a small amount, the concentration of dissolved carbonate ions falls. When that happens, biogenic calcium carbonate becomes more soluble and can start to dissolve. Depletion in dissolved carbonate ions thus makes it harder for such organisms to maintain their protective or skeletal structures. In the worst case scenario, the rate of calcium carbonate dissolution is faster than its formation. When that happens, mass-mortality of calcifying organisms can occur.
We're talking about critters that underpin entire marine food-chains here. Things from near-microscopic calcifying plankton to shellfish, lobsters and crabs the seafood we eat in other words. That's why ocean acidification is deadly serious.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
Not all of the CO2 emitted by human industrial activities remains in the atmosphere. Between 25% and 50% of these emissions over the industrial period have been absorbed by the world’s oceans, preventing atmospheric CO2 buildup from being much, much worse. But this atmospheric benefit comes at a cost.
As ocean waters absorb CO2 they become more acidic. This does not mean the oceans will become like the acids one encounters in a chemistry lab. However, marine life can be highly sensitive to slight changes in pH levels and any drop in pH is an increase in acidity, even in an alkaline environment. Worse, the pH scale is logarithmic, meaning that for each single-digit decline in pH, acidity (defined as hydrogen ion activity) rises tenfold.
Surface seawater pH has been relatively stable over recent geological time, fluctuating between cold glacial periods (pH 8.3) and warmer interglacials (pH 8.2). But since the Industrial Revolution, average seawater pH has dropped towards a recent figure of less than 8.06, an approximately 30% increase in acidity (fig. 1). This is a faster change than any over the past 50 million years (Rhein et al, 2013, available from IPCC here).
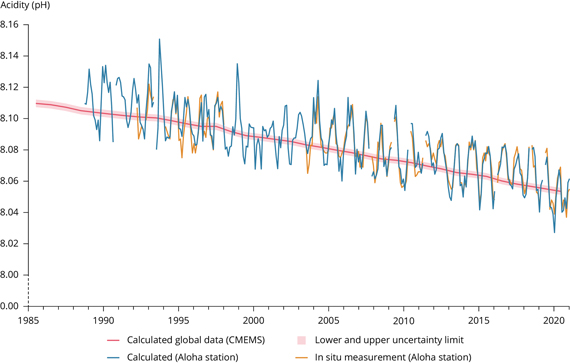
Fig. 1: Decline in ocean pH measured at the Aloha station (in the Pacific Ocean off Hawaii) and yearly mean surface seawater pH reported on a global scale Source: European Environment Agency (Copernicus Marine Service).
Because of its inextricable link with CO2 emissions, this rate of acidification is projected to accelerate even further through the 21st century under a business-as-usual scenario with potentially catastrophic impacts to marine ecosystems (Bindoff et al. 2019 (PDF from IPCC)). These trends are becoming clearer globally.
According to the IPCC's Sixth Assessment Report (AR6), there is, " a very likely rate of decrease in pH in the ocean surface layer of 0.016 to 0.020 per decade in the subtropics and 0.002 to 0.026 per decade in subpolar and polar zones since the 1980s. Ocean acidification has spread deeper in the ocean, surpassing 2000 m depth in the northern North Atlantic and in the Southern Ocean (fig. 2)."
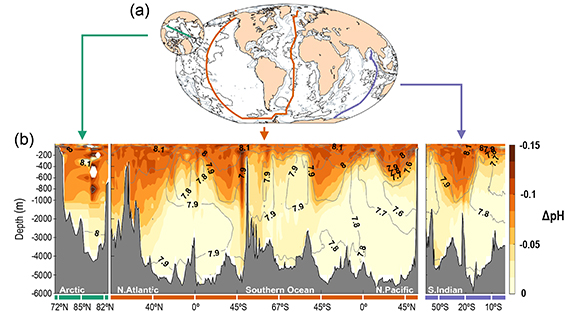
Fig. 2: Spread of ocean acidification from the surface into the depths since pre-industrial times. (a) Map showing the three transects used to create the cross sections shown in (b), showing the vertical sections of the changes in pH between 1800–2002 due to anthropogenic CO2 emissions; the darker the colours the greater the change. Contour lines are their contemporary values in 2002. Graphic sourced from IPCC AR6. (Lauvset et al. 2020).
Such changes in ocean chemistry, if allowed to occur, will be irreversible for many thousands of years. The biological consequences could last much longer.
How do we know that? Through the geological record. When mass-extinctions have occurred, most of them are tied-into unimaginably severe episodes of volcanism, at a scale never witnessed by humans. But the carbon footprint of such cataclysms has in fact been similar to our own. And what do we see as a consequence of such events? The fossil record shrinks in terms of its biodiversity and there are what we call 'reef-gaps', periods of several million years during which coral reefs - large highly diverse colonies of corals and myriad other species - were to all intents and purposes absent.
The reason why reef-gaps occur at such times is because as surface waters become more acidic, it becomes more difficult for corals, shellfish and other calcifying organisms to form and maintain the hard calcium carbonate skeletons or shells necessary for their survival. When things start getting really bad, that calcium carbonate dissolves away as fast as it can be deposited - that means curtains for such critters.

Fig. 3: just some of the life-forms at deadly risk from the acidification of near-surface ocean waters.
Coral reefs provide a home for more than 25% of all oceanic species, so you can see why this matters so much. Some calcifying organisms, such as the tiny pteropods (fig. 3), underpin many oceanic food chains: take them out of the system and down those food-chains come crashing. Many communities around the world, constituting millions of people, are at the apex of such food-chains, relying on seafood as part of a healthy diet. You should now be able to see the problem. Like a thief in the night, ocean acidification is creeping up on us, while we sleep on in blissful unawareness.
Last updated on 25 June 2023 by John Mason. View Archives































 Arguments
Arguments




































[DB] FYI, posting of links is allowed provided you also explain the context of the link and why it matters/why it's germane to this discussion. Also, there is a robust body of evidence extant in the peer-reviewed, published literature. If you wish to be credible, please draw support from that body with links to papers supporting your position/the point you're trying to make.
Congressional testimony alone is not credible in a scientific forum such as this one.
Future comments constructed such as this one will typically be deleted.
holoman @60
The link you provides shows only that the Thomas Institute for Technicology Research (an organisation that today hardily exists on the interweb where its history stretches back all of five days, an organisation that is not prepared to give the slightest indication of who or what or where it is) has access to a chemistry book. Thus they bravely tell us - CO2 (aq) + H2O <>H2CO3 <>HCO3− + H+ <>CO32− + 2 H+ or in english In aqueous solution, carbonate, bicarbonate, carbon dioxide, and carbonic acid exist together in a dynamic equilibrium.
So would you/can you give further explanation?
[RH] Holoman made a "link only" post and needs to repost the link with some relevant discussion.
Here's a very annoying "oh yeah?" question I got about the issue of ocean acidification, warming and corals (and other shelled organisms). The dude basically said, "Oh yeah? Well if these things are so dangerous to corals, how did corals survive earlier periods when the ocean was much warmer and more acidic than it is now?"
Can anyone answer that?
[Rob P] - Sorry, I have written a series of rebuttals on this very topic but need to get our SkS graphics guru to create the animations necessary to convey the essential points.
Firstly, it's the concentration of carbonate ions that is important for shell-building. Tom Curtis is not quite correct about calcium ions. Both are the building blocks of the calcium carbonate structures, but calcium is largely invariant on sub million-year time scales. This is not the case for carbonate. So it's the concentration of carbonate ions that is the issue. A measure of the availability of carbonate/calcium ions is called the calcium carbonate saturation state - aragonite (a form of calcium carbonate) in the case of reef-building coral.
When additional CO2 is added to the atmosphere, more of it dissolves into the surface ocean. One of the reactions that takes place is the conversion of carbonate to bicarbonate when the carbonate ions combines with a hydronium ion. This buffers the decline of pH at the expense of the calcium carbonate saturation state, i.e. pH would fall even further if not for this reaction.
At equilibrium, CO2 is introduced into the atmosphere by volcanic activity and removed by the chemical weathering of rocks. If these two were not in balance, the climate would inexorably drift without any external influence. With geologically-rapid injections of CO2 into the atmosphere, the carbonate system is overwhelmed. The warmer atmosphere brought about by the extra CO2 increases the moisture content of the air and the greater rainfall-induced weathering (and dissolution of carbonate sediments on the ocean floor) flush more carbonates and bicarbonates back into the ocean, thus restoring the saturation state. This process takes tens to hundreds of thousands of years though.
So ocean acidification only occurs with geologically-rapid injections of CO2. With slow increases, or steady states, the weathering response is able to supply alkalinity back to the ocean to compensate for the carbonate ions converted to bicarbonate. Actually, the tendency is for the weathering process to overcompensate - creating an ocean carbonate saturation state that is even more hospitable to calcification than before the acidification event, or slow increase in atmospheric CO2.
With this understanding, the geological record now makes sense. Extinction events, such as the End Permian extinction (where 95% of marine life was extinguished), involved a large and geologically-rapid increase in CO2 and therefore experienced ocean acidification as a kill mechanism. Marine life that were vulnerable due to their carbonate mineralogy were preferentially extinguished. Periods of sustained high atmospheric CO2, on the other hand, did not cause a calcification crisis (although tropical ocean warming was an obstacle) because the carbonate saturation state was much higher than today. A classic example is the Cretaceous (Latin for chalk - as in calcium carbonate shells) Period, named after the prolific shell-formation that accumulated during this interval. Atmospheric CO2 was high and ocean pH was low, but the ocean carbonate saturation state was very high. The ocean back then was therefore very hospitable to calcification.
So it's only rapid CO2 increases that cause ocean acidification. And we just so happen to be increasing Earth's atmospheric carbon dioxide concentration faster than it has ever increased in the last 300 million years. Very serious indeed.
dvaytw, 'How did they survive'? The simple answer is... they didn't. One of the primary reasons we know that these changes are going to kill corals (aside from the fact that it is, you know, already happening) is that there were coral die offs from similar events in the past.
It is also important to note that the rate of change is often more important than the absolute value. Changes which occur over the course of thousands of years allow organisms some time to adapt. Those which occur over the course of only centuries or decades generally do not.
dvaytw @61, that is a simple question requires a moderately complicated answer.
The most fundamental point is that ocean acidity is controlled not only be pCO2 concentrations, but also by Calcium ion concentrations. A high dissolved CO2 concentration can be largely offset by a high Calcium ion concentration. Calcium is introduced to the ocean by the weathering of rocks, both by rain and plant activity. Both of those increase in a warm (high CO2) world so that a natural tendency to equilibrium exists. The result is that even in the eras of highest CO2 concentration over the past 500 million years, ocean pH has not fallen below 7.4 (Zeebe 2012, see figure 5). Past increases in CO2 concentration have been very slow in comparison to the modern anthropogenic increase, allowing ocean chemistry to adjust and restrict the resulting rise in ocean acidity. Because the modern increase is so fast, however, the calcium buffer is being exhausted, allowing a far higher ocean acidity relative to atmospheric CO2 concentration than has been the case in the past.
Second, it is not expected that increased ocean acidity will kill all corals. In particular, soft corals are not directly effected by ocean acidification, and can be expected to flourish (as will other related animals such as sea anenomes as jelly fish). Given high ocean acidity, hard bodied (ie, reef building) corals may revert to soft bodied forms and potentially survive in that way. The consequence of this, however, is that the reefs themselves will decay and be destroyed. The reefs form the basis of major ecosystems, and if they are destroyed most of the reef fishes will find themselves without habitat (including many of the newly soft bodied corals). The consequence will be that many of the reef species will go extinct, and those that survive will do so by adopting a non-reef mode of life - becoming much rarer than is currently the case. The human impact will be a reduction of the shelter provided to tropical shores by reefs, and a great reduction in tropical fisheries.
Third, as CBDunkerson points out, modern corals (scleractinia) have only existed for the last 220 million years, and missed the periods of very high CO2 levels in the past. Indeed, the leading explanation for the supplanting of previous reef building corals is that soft bodied ancestors of modern corals survived, where their hard bodied rivals did not, during the very high CO2 episode of the Permian extenction, and then evolved in to the thus vacated ecological niche. Since then, hard bodied forms of scleractia (and the reefs they produce) have disappeared several times in periods of high CO2 concentrations, ony to reappear several million years later. That several million year delay suggests to me that the scleractia have had to reevolve the reef building habit, ie, that the survivors were not the reef builders of the period but their soft bodied cousins. In any event, the last reappearance was 40 million years ago, when ocean acidity dropped to levels lower than can be expected from BAU over the next two the three hundred years (or one hundred if we are determined).
What is wrong with a lower pH? It seems to me it would be a good thing if we could increase our CO2 to 2000 ppm. The ocean pH would probably drop to around 7.4, which is physiological conditions.
Seems to me that the catastrophic event has already happened. The catastrophe happened when the CO2 dropped to 180-280 ppm from 1000-4000 ppm, which was the concentration of CO2 when invertibrate life evolved on this earth.
The pH of the ocean is directly related to the atmospheric CO2 concentration. The pH was obviously at 7.4 when invertibrates evolved because our enzymes don't function at a different pH. Organisms have evolved to maintain a pH of 7.4 despite the fact that our environment no longer has that pH. Seems ridiculous to worry about restoring that pH. There may be a few organism that get outcompeted by the burst of biodiversity that would be almost certain to occur if we return to the non-hostile environment of 1,000-4,000 ppm CO2.
[Rob P] - The rate of change seems to be the key issue. See here: Why were the ancient oceans favorable to marine life when atmospheric carbon dioxide was higher than today?
The image below (also included in the linked blog post above) perfectly demonstrates how the actual experts have a far better handle on this than you. Both fossils date from ancient periods when atmospheric carbon dioxide was much higher than now, but only the fossil on the right lived in a time of ocean acidification. The geologically-rapid, but many times slower-than-present, increase in atmospheric CO2 during the Paleocene-Eocene Thermal Maximum drove carbonate undersaturation of the surface ocean.
I'am a scientist, but not a climate scientist. I don't understand why climate scientists don't ask and answer more unbiased questions. For instance, regarding the pH of the ocean, shouldn't you be trying to figure out what the optimal pH of the ocean is. A good scientist would ask questions like: What pH would create the most biodiversity?, or What pH would sustain the most food for humans?, or What pH would sustain the most total mass of ocean life?, or Which species would benefit most from a drop in pH?
This kind of unbiased science allows government policy makers to make informed decisions. We need all the facts on the table. If the scientist starts with a political agenda in mind, he or she robs society of the opportunity to decide the policies that are best for society. The scientist becomes the judge, jury, and prosecution.
Scientists are supposed to be trained to avoid political bias in their research. The peer review process is suppose to ferret out political bias. I'm not sure what happened with climatology, but the peer review process seems to have failed us in this particular field.
[GT]
Violations of site policy blocked. Get some more knowledge before you throw around accusations. At present you just look a little foolish.
Andrew1776.
Firstly, what field of science are you in, because from your comments it obviously isn't ocean chemistry.
Next, pH itself isn't the primary consideration, that is just an indicator of the bigger issue which is the change of carbonate chemistry linked to changes in CO2 and pH and the flow on consequences for shell forming marine life that use calcium carbonate in forming their shells.
If you haven't done so already I would recomend you read the intermediate level of this rebuttal, then follow that up by reading the OA is not OK series, linked from the side-bar on the upper left. Only then will you begin to know enough to understand how unihformed your comments are.
Next, even in the hypotheal that we could change the pH in ways you suggest, it would seem naive to assume that this would then be beneficial. Marine organisms have evolved to live in a vastly complex chemical environment where pH is a contributing factor in a wide range of chemistry. Changes in pH may be positive or negative, that is a very open question.
Additionally, making major changes in ocean pH requires major disturbances such as we are currently experiencing. Given sufficient time (this might be centuries to millenia) pH will return to previous levels. This is part of the complex chemistry you don't seem to know much about.
So for example, this statement of your is simplistic
"The pH of the ocean is directly related to the atmospheric CO2 concentration".
Also to the concentration of boric acid in the ocean for example.
So before making any further comments, might I suggest you get a lot more knowledge first. Then come back and revisit your comments.
Because currently your last 2 paragraphs in your second post are violations of the comments policy here. Your apparent lack of knowledge may have led you to make them but the other insinuations of political bias etc are out of order. Nothing went wrong in 'climate science'. You are just commenting with insufficient knowledge. Go get some.
Glenn Tamblyn
Thanks for the response. My field is materials science. I have a fair amount of experience with cement chemistry, which as you may know involves calcining limestone (CaCO3) and creating calcium silicate hydrates that precipitate to form cement. The chemisty is actually quite complex. You may not know this, but Le Chatlier was a cement chemist and he described the Le Chatlier priniciple in his PhD dissertation on cement hydration.
So yes I get the chemistry. And no I didn't find the "OA not OK" series to be helpful. Most of what I saw was just a review of basic acid/base chemistry (no pun intended) =). The "peanut throwing" example is quite below my level of understanding. Perhaps it is helpful for people without a chemistry background.
I'm intersted in finding real data on the optimal pH for for coral. Coral evolved when the oceans had a pH of 7.4. My theory is that lower pH should be good for the vast majority of coral (perhaps pH 7.4-7.8).
I did a google search for "coral mineralization". The first article that turns up is Bionature 2011. It says the rate limiting step of coral mineralization is CO2(aq) + H2O CaCO2. In other words, Coral production is limited by the amount of CO2 dissolved in the ocean. Which is what you expect. CO2 is the raw material for making Coral. Increasing its concentration should have a beneficial affect. Duh, no?
So how do climatoligist come to the opposite conclusion? I'm not exactly sure, but it seems to be related to the fact that rising temperatures reduce the solubility of CO2. So theoretically, if the temperature rises, there would be less dissolved CO2 in the ocean and it would harm the coral. Of course that isn't happening. The pH of the ocean is lower, not higher. And you can have it both ways. You can't say that the CO2 is going to harm the Coral because the temeprature is going to be higher and reduce the CO2 and use that as a justification why increasing CO2 is bad. If the pH is lower, it can't be higher. The logic is just wrong.
Correct me if I'm wrong, but I'm not aware of any actual evidence that higher temperature is bad for Coral appart from the risk of less CO2 in the water. So, given the ample data we have that pH of the oceans is dropping, the temperature doesn't matter. The Coral should be healthier in years to come because of lower pH.
I didn't see anything about boric acid. Perhaps I'll dive into that another night. I'm sure it is just like everyting else I've seen...all the data points to CO2 having a beneficial effect on planet. If anything, the planet is sick right now due to lack of CO2.
Believe it or not, I'm an environmentalist. But every time I look at the raw data it suggests that CO2 is good for the environment. I've come to this forum because I'm at a complete loss as to how climatoligists think CO2 is bad. It's plant food. What's not to like about feeding the plants?
[Rob P]
It's a shame you don't understand the peanut-throwing analogy, because it explains perfectly why your non-expert intuition is wrong here. Once equilibrium shifts toward dissolution the coral polyp has to expend more energy to build its aragonite skeleton. A number of lab experiments, in published papers, seem to bear this out.
It's the availability of carbonate ions in the calcifying fluid that is important. The polyp raises pH in order to supersaturate the calcifying fluid and allow aragonite crystals to precipitate. By lowering ocean pH we're making it harder for the coral polyp to build its skeleton.
Rob P
I'm interested in your thoughts on rapid rise in CO2 causing mass extinction. You say that CO2 was high during the crestacean period, but then you say it was a rapid rise in CO2 that caused the catestrophic events of the K/Pg boundary. How did it rapidly rise if it was already high?
Andrew1776, please revise your spelling as you go along. The dianasaurs of the crestacean period are extinct - but they deserve some respect, all the same !
Like 1776, your ideas are certainly revolutionary (but not in any way evolutionary). You seem to be suggesting (against the evidence) that the marine life forms will benefit from lower pH and a much higher pH-logiston level. Or some similar eighteenth century level of concepts.
Andrew, there have been a great many advances in science since 1776. You should embrace these advances, rather than reject them.
[PS] please stick to comments on content not grammar or style.
Eclectic,
While your comments have the appearance of politeness, they are ad hominems. Where are the moderators on this?
More importantly, you don't address the issue. Do you agree that life evolved when the oceans were pH 7.4? We call this "physiological conditions". My premise is that it should be good for the planet for man-made CO2 to restore the earth to physiological conditions.
Climatology produced numerous papers (several in high profile journals such as nature) saying that carbonate-based organisms would be harmed by increasing CO2. Yet these organisms evolved in a high CO2 environment. It just doesn't make sense. Climatology produces "models" saying the coccolithophores are going to be destroyed, but then when someone actually measured what has happened to coccolithophores as pH has decreased, it turns out they increased by 10 fold. see: http://hub.jhu.edu/2015/11/26/rapid-plankton-growth-could-signal-climate-change/
Of course the original theory was catastrophic harm to coccolithophores. Now the problem is catestrophic success.
[PS] Fixed link. Please provide a link to support that statement "Climatology produces "models" saying the coccolithophores are going to be destroyed".
Andrew1776 @70 and prior posts (including recently in other threads) ,
you fail to recognize or acknowledge that land-based and sea-based life forms have diverged in their evolution for 100's of millions of years (regarding body chemistry). In your passionate desire for marine creatures to make a problem-free transition to a low pH (or high phlogiston) condition in an eye-blink of evolutionary time, you are (it seems) indulging in wishful thinking of the most unrealistic kind.
In short, your revolutionary and idiosyncratic idea of an unproblematic abrupt change in physiological conditions , is an idea which comes several hundred million years too late.
Please remember that we a playing for high stakes - and the rapid "unnatural" acidification is a matter involving the entire planetary ocean : not a micro-experiment on a saline gallon or two in a kitchen sink. The high stakes require an intelligent risk-management approach to the situation, don't you agree?
An approach based on extensive biological knowledge, rather than on poorly-informed caprice.
I find that it makes as much sense to say that CO2 is plant food than to say that O2 is people food. It is intellectually dishonest and physiologically inaccurate.
Andrew1776 @67 cites Rahman and Shingjo (2011) as stating that "... the rate limiting step of coral mineralization is CO2(aq) + H2O CaCO".
With regard to that claim, it should first be noted that what is produced by Andrew1776 is not a valid chemical formula, something somebody with his claimed expertise should know. The correct formulas are:
Equation (2) represents the rate limiting equation, but that is not the whole story. It is equation (5) that represents the production of calcium carbonate. The crucial compound whose abundance controls the final rate of production of CaCO3 is CO32-. The ratio of the various reaction components in equations (2) through to (4) is determined by, among other things, the acidity of the water. It is shown on this graph (note the logarithmic scale):
You will notice that with the expected decrease in pH by the end of this century wiht BAU, the proportion of CO32- falls by more than 50%. That is just the ratio to the other compounds, of course, and as Andrew1776 is keen to point out, CO2(aq) will rise, and the relative amount of the other compounds with it. In fact, with business as usual, it will approximately double:
The net effect is that the absolute quantity of CO32- will fall, and with it the rate of calcification. That is in addition to any direct adverse effects from acidification.
Further to my post @73, regardless of the theoretical basis, it is pointless for Andrew1776 to argue that increased CO2 levels would be good for corals, for too many real world examples exist proving the contrary. In particular, examples such as the CO2 seeps at Milne Bay:
Note that changes from pH factors typical of those expected in open ocean around 2050 (a) to those expected by the end of the century (b) with BAU do not reduce the coral cover, but massively reduce the diversity of coral species. In particular:
Note that the reduction in juvenile Porites (> fourfold) shows them also to have been adversely effected by the increased pH, but that the lack of competition from other corals allow an overall increase in adult forms.
The sites with pH levels expected in the next century with ongoing BaU (c),
More detail about the specific effects can be found here.
Similar observations have been made at other CO2 seeps in New Guinea, and the Mariana Islands, among others.
So, the fact that elevated pH due to increased CO2 concentration adversely effects corals and other calcifying sea life is not just a matter of theory, but of direct observation. Observation that Andrew1776 wants to simply ignore.
[PS] Since comprehensive literature review is a better indicator of state than cherry picking a paper, I note this extensive review recently published here.
[JH] Recommended supplemental reading:
Ocean acidification: climate change's evil twin by Lars Bevanger, Deutsche Welle (DW), Nov 21, 2017