Is CO2 a pollutant?
What the science says...
| Select a level... |
 Basic
Basic
|
 Intermediate
Intermediate
|
 Advanced
Advanced
| ||||
|
A single substance can be both a pollutant and a non-pollutant. It all depends on context. |
|||||||
Climate Myth...
CO2 is not a pollutant
'To suddenly label CO2 as a "pollutant" is a disservice to a gas that has played an enormous role in the development and sustainability of all life on this wonderful Earth. Mother Earth has clearly ruled that CO2 is not a pollutant.' (Robert Balling, as quoted by Popular Technology)
At a glance
If you look up the definition of pollution in a dictionary, you will soon realise it's rather subjective. There are many substances out there that are harmless at certain levels but harmful at others.
Carbon dioxide is well-mixed in our atmosphere. That's because when it is emitted, by any mechanism from a vehicle exhaust to a volcanic eruption, it stays in the air for many years. Unlike water, it does not condense and fall back out as rain. Turbulence does a splendid job of mixing it evenly into the air. But there are places on - and in - Earth where much higher concentrations of CO2 may be encountered.
The trouble with CO2 is that it cannot be seen and neither can it be smelt. In other words we cannot detect it from a safe distance.
In caves and mines, high concentrations of CO2 are a well-known hazard. They can result from things like rotting timber, oxidising coal and particularly by poor ventilation, where that mixing into the air fails to occur. Because CO2 is heavier than air, in poorly ventilated areas underground it may collect into pockets waiting for the unwary.
Miners or underground explorers breathing a higher than normal concentration of CO2 will experience gradually increasing ill effects. It depends on the concentration of the gas. For example the UK Health and Safety Executive has defined safe CO2 limits for the workplace. The limit for long-term exposure is 0.5% (5,000 ppm) but for shorter encounters it is 2%. Anything over that figure is regarded as a risk to human health. There have been many accidents and fatalities over the years caused by high concentrations of CO2 in underground workings and to a lesser extent in caves. Coal-miners refer to CO2 as black- or choke-damp in recognition of the hazard.
Possibly the worst CO2-related disaster was that of 21 August 1986 at Lake Nyos, in northwestern Cameroon in western Central Africa. The lake, only some 2 x 1 km in size but more than 200 m deep, is one of a number of flooded volcanic vents in a sporadically-active volcanic belt. Carbon dioxide-bearing springs are common in this area and some are present in the lake-bed.
Lake Nyos is typically stratified, meaning that normally its waters occur in distinct layers with different chemistry that do not normally mix. In something of a loaded gun scenario, the bottom layer used to become saturated with CO2 from those lake-bed springs. On 21st August 1986, something caused an overturning of the lake, meaning the deep CO2-saturated water headed for the surface. Like taking the top off a shaken-up pop bottle, a vast cloud of CO2 was instantly released and travelled out from the lake along the ground. At least 1,746 people and 3,500 livestock died instantly from asphyxiation.
Modern technology and international cooperation have since been successful in controlling the build-up of CO2 in lakes like Nyos. But clearly, in specific circumstances, CO2 is as deadly a pollutant as any other.
Please use this form to provide feedback about this new "At a glance" section. Read a more technical version below or dig deeper via the tabs above!
Further details
We commonly think of pollutants as contaminants that make the environment dirty or hazardous to Nature and humans. A vivid example is sulphur dioxide, a common by-product of industrial activity. High levels of sulphur dioxide cause breathing problems. Too much SO2 causes acid rain, because it is so highly water-soluble. Sulphur dioxide has a direct effect on health and the environment. Fortunately, it reeks and so can be detected by us quickly, at concentrations as low as 1 ppm.
Carbon dioxide, on the other hand, is a naturally occurring gas that existed in the atmosphere long before humans. Plants need it to survive. The CO2 greenhouse effect keeps our climate from freezing over. These are all popular talking-points that any climate change denier will rattle out in its defence. How on Earth, they say, can CO2 be considered a pollutant?
Well, CO2 is very definitely a hazardous substance when it collects due to being denser than air or suddenly invades an environment in large quantities. Under either scenario it is capable of instant asphyxiation of any living thing with the bad luck of being in that place. The Lake Nyos disaster of 21st August 1986 (Tanyileke et al. 2019) is probably the most notorious example. A stratified lake - where the waters usually do not mix - underwent sudden overturn. The overturn brought pressurised CO2-saturated deep water up to the surface in an explosive release. It sent a cloud of CO2 - estimates vary but it could have been as much as a billion cubic metres - hurtling over the lake's rim. The gas cloud swept outwards at an estimated 72 kilometres per hour. In its path were several villages; the most distant to be affected, Mashi, was some 20 kilometres from the lake. At least 1,746 people and 3,500 livestock were asphyxiated.
If you explore mines or caves, or work in them, you will be well aware of the lethality of isolated high concentrations of CO2. These typically occur in badly or non-ventilated areas. Here, sources of CO2 such as an underground mineral-spring or rotting timbers, are not buffered by mixing-out of the gas in air. Instead, the gas can lurk in pockets and is a lethal hazard to the unwary. The experienced carry portable meters that constantly read the concentration of oxygen and other gases. Thus they can provide immediate warning that a pocket of 'bad air', as it's known, has been entered.
So under certain circumstances, CO2 is very definitely noxious to say the least.
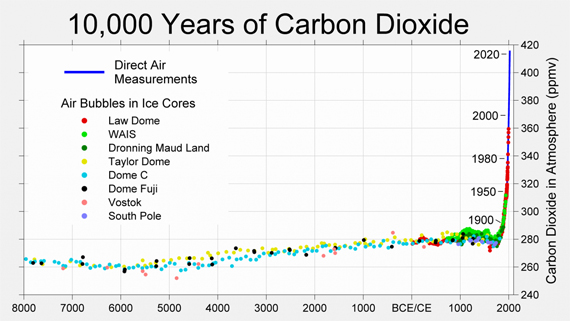
What about the effect of raising atmospheric CO2 levels? Over the past 10,000 years, the concentration of carbon dioxide in the atmosphere has remained at relatively stable levels of around 280 ppm. However, human CO2 emissions over the past few centuries have upset this balance. Since industrial times started, CO2 levels have risen to over 420 ppm - a 50% increase (fig. 1).
The increase in CO2 due to human emissions has direct effects on the environment. For example, as the oceans absorb increased CO2 from the atmosphere, that leads to acidification. Acidification affects marine ecosystems. It can lead directly to mass-mortality of calcifying organisms. In other words it can effectively destroy oceanic food-chains. Whole fisheries may disappear as a consequence.
Figure 1: CO2 levels (parts per million) over the past 10,000 years. Source: Berkeley Earth.
Last but by no means least is the impact from rising CO2 in the form of warmer temperatures. Rising CO2 levels cause an enhanced greenhouse effect. This leads to warmer temperatures which have many consequences. Some effects are beneficial such as improved agriculture at high latitudes and increased vegetation growth in some circumstances. However, the negatives far outweigh the positives. Coast-bound communities are threatened by rising sea levels. Melting glaciers threaten the water supplies of hundreds of millions. Species are becoming extinct at the fastest rate in history.
How we choose to define the word 'pollutant' is a play in semantics. To focus on a few positive effects of carbon dioxide is to ignore the broader picture of its full impacts. The net result from increasing CO2 are severe negative impacts on our environment and the living conditions of future humanity.
Last updated on 27 August 2023 by John Mason. View Archives































 Arguments
Arguments




































Regarding lag, see "CO2 lags temperature."
Regarding carbon sequestration by agriculture--yes, it has some unrealized potential and most definitely is being considered; see "It’s too hard."
Also, see "It’s ozone."